HYDROBORATION-OXIDATION OF ALKENES
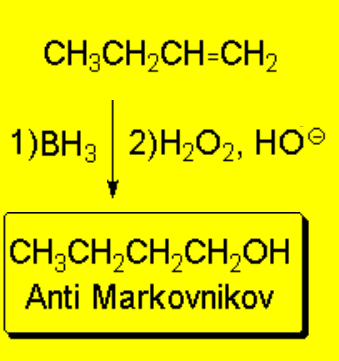
The hydroboration of olefins, followed by oxidation, allows us to obtain alcohols with
anti-Markovnikov
regiochemistry.
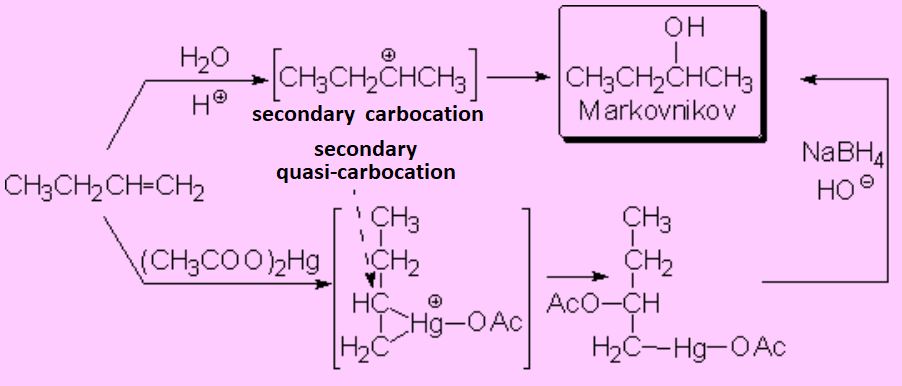
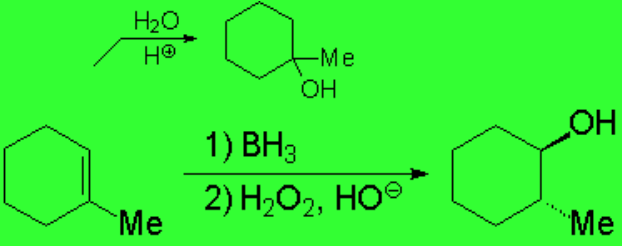
The addition of water to olefins or their oxymercuration-demercuration reactions proceeded by carbocations or quasi-carbocations, respectively, leading to a Markovnikov regiochemistry.
Therefore, the fact that the hydroboration-oxidation of olefins produces anti-Markovnikov alcohols implies that this process must take place by an entirely different mechanism, without the concourse of carbocations:
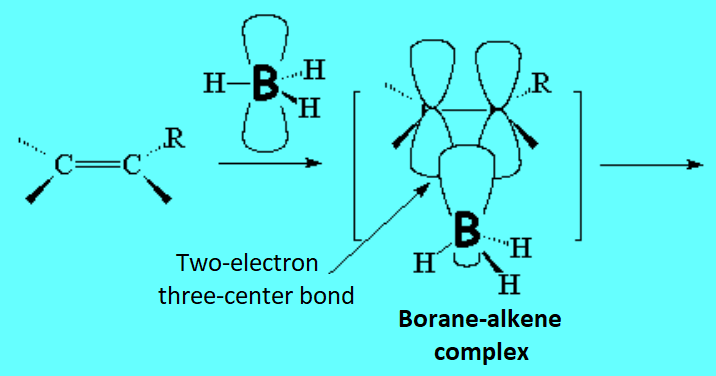
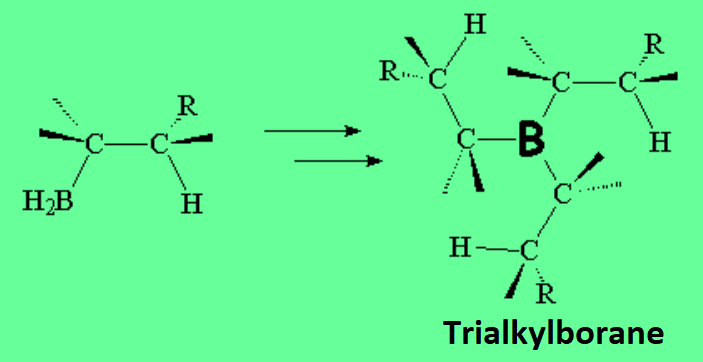
The first step is simply an acid-base Lewis reaction, between the "pi" cloud of the olefin (base) and the boron atom of the borane (acid) that bears an incomplete electron octet.
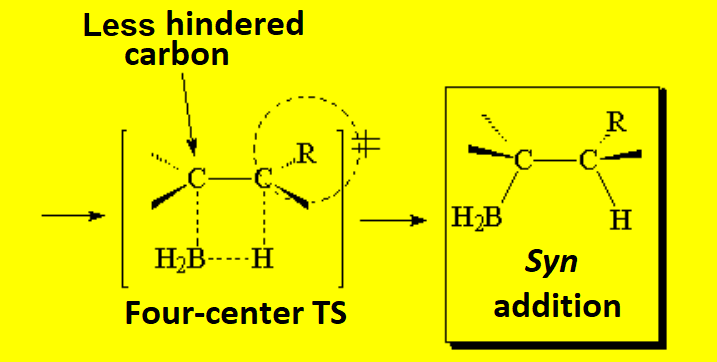
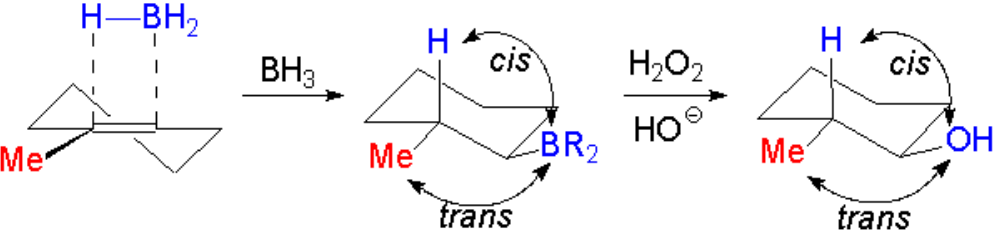
In the second step, the complex borane-alkene breaks up and the boron shifts to the least substituted, hindered carbon.
This is key in achieving the anti-Markovnikov alcohol because the OH ends up where the B was.
Once the alkylborane is formed, the boron atom again bears an incomplete octet and that makes it possible for B to react with two additional moles of olefin.
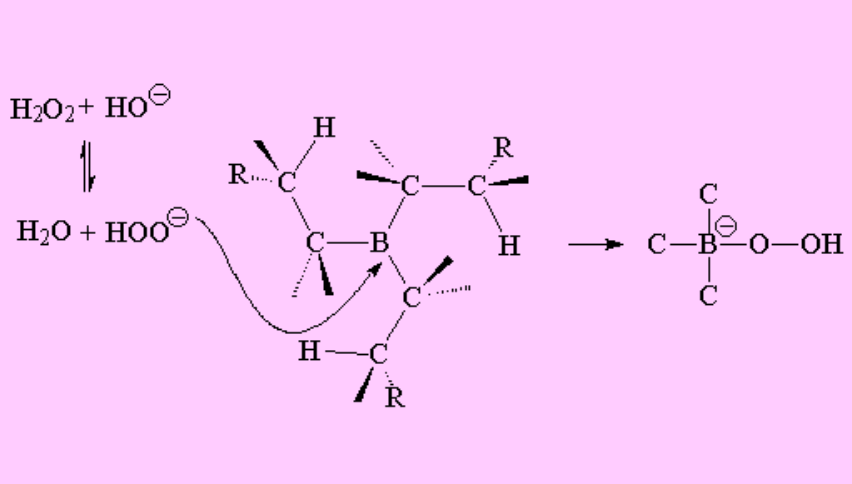
The following steps belong to the oxidation phase where the trialkylborane reacts with oxygenated water in basic media.
In the trialkylborane, the boron atom keeps bearing an incomplete octet but it does not possess any hydrogens to add to another olefin.
However, the boron atom admits electrons from the oxygenated water deprotonated by the hydroxide.
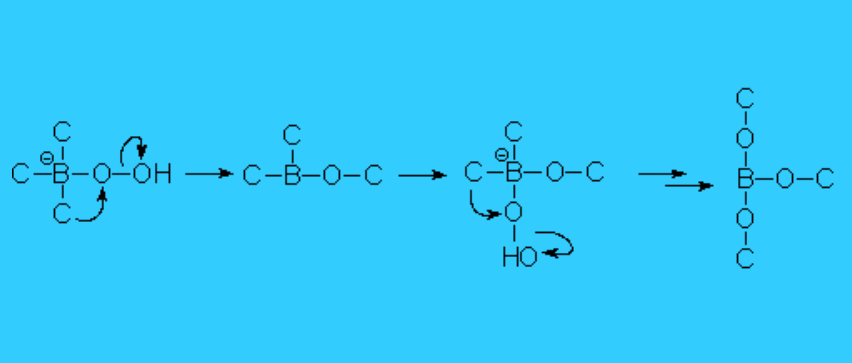
Carbon then rearranges from the boron to the oxygen, with the loss of hydroxide, that is thus recovered.
The weakness of the O-O bond makes the carbon rearrangement possible.
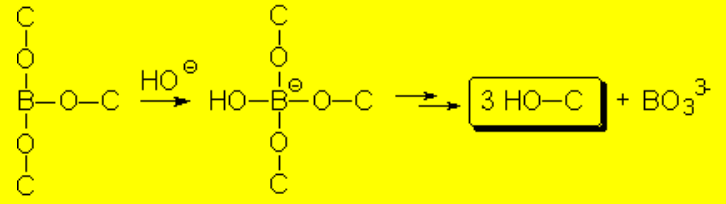
The alkoxyborane is hydrolized helped by the basic madium leading to the alcohol and borate.
The reaction is regioselective and stereospecific:
The regiochemistry is anti-Markovnikov and the stereochemistry of the addition is syn.
That's why the OH and the Me groups end up in trans arrangement in the example.
The boron atom is replaced by the OH with retention of the configuration.