 |
|
The face of the olefin at the opposite side to the cylopropane ring can easily reach the catalyst surface without any notable hindrance. Hence, hydrogenation takes place much faster by this face, leading to cis-carane, which is the least stable of the two possible isomers. Trans-carane cannot be produced even though it is the most stable because it lacks the signaled steric interacton beteen the methyl groups. The approach of carene to the catalyst to render trans-carane is heavily hindered by one of the two methyl groups attached to cyclopropane ring.
The qualitative diagram of reaction energy should be similar to the following:
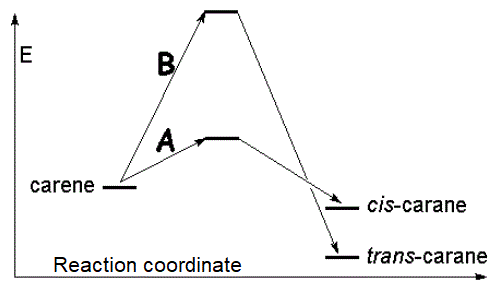 |
Pathway B has a much larger activation energy and proceeds at a much slower pace, even though it produces the most stable trans-isomer.
The cyclopropane ring fused with the ciclohexene carries two stereogenic centers of fixed configuration. This fact makes the two faces of the double C=C bond have very different surroundings.
The difference is so large in the case of carene and the molecule is so rigid that the hydrogen only adds to one of the two faces giving rise to a total facial selectivity.