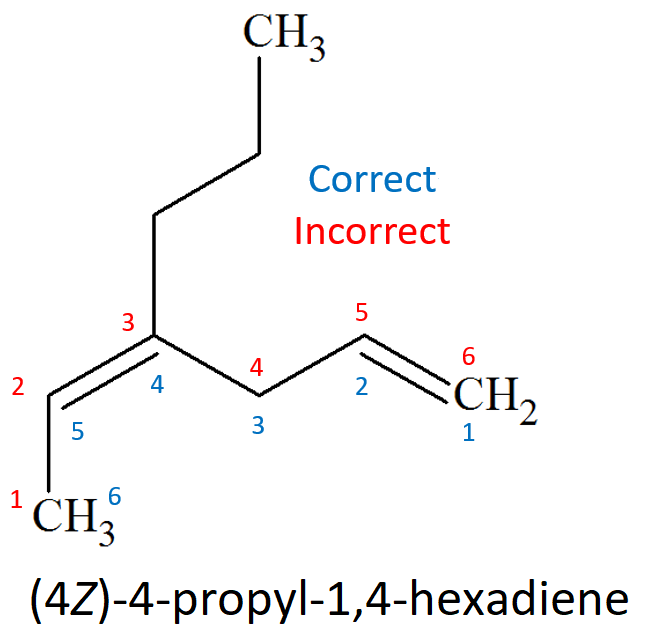
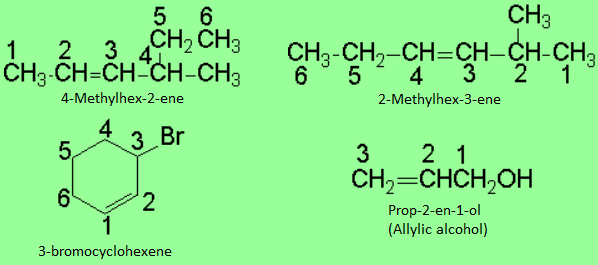
The chain must be numbered in such a way that the carbons of the double bond should end up with the lowest possible localizing numbers, exception made when there is an additional dominant function.
If substituents are present, they must bear the lowest possible localizing numbers.
In the case that one ends up with two equivalent double-bond numberings, that with the lowest one for the substituents must be taken.
In cycloalkenes, the numbering starts by the double bond and it is continued in order to assign the lowest possible numbers for the substituents, if any.
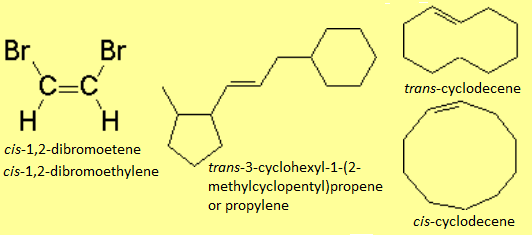
In disubstituted alkenes, the cis-trans or Z/E stereochemistry must be specified at the beginning of the name.
In disubstituted alkenes, in addition to the "classical" cis-trans stereochemical nomenclature, the Z/E prefixes can be used, from the german E=entgegen (far away) and Z=zusammen (together).
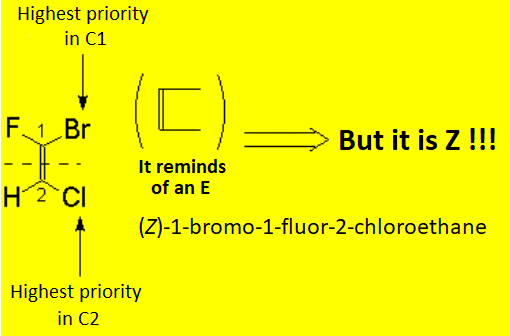
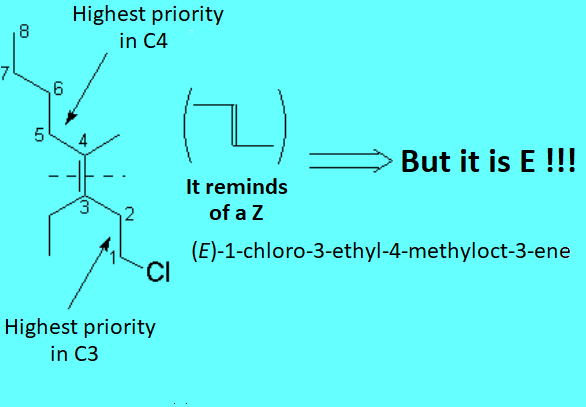
There is no other choice in the case of tri- or tetrasubstituted alkenes. The substituent priority rules used at bond ends of the olefin, in order to define whether the most important radicals are far away (E) or together (Z), are the same as those applied to R/S nomenclature.
The following "joke" may be useful to remember when an olefin is E or Z.
You know that the moon is a liar:
Crescent when it is in D shape, and Decreasing when in C shape.
Alkenes are as liar as the moon is because when the most important substituents at both ends of the double bond are in Z shape, the olefin is E and viceversa.