As compared to a single C-C bond, the double C=C bond bears a planar geometry, shorter bond lenghts and higher bond energy.
The C(sp3)-C(sp2) bonds have a slight dipole moment because the olefin sp2 carbons are understood to be slightly more electronegative than the alifatic sp3 ones. This is explained due to the higher s character of the sp2 hybridization (one third) as compared to the sp3 (one quarter). The electrons in a sp2 orbital are slightly closer to the atomic nucleus and hence more attracted.
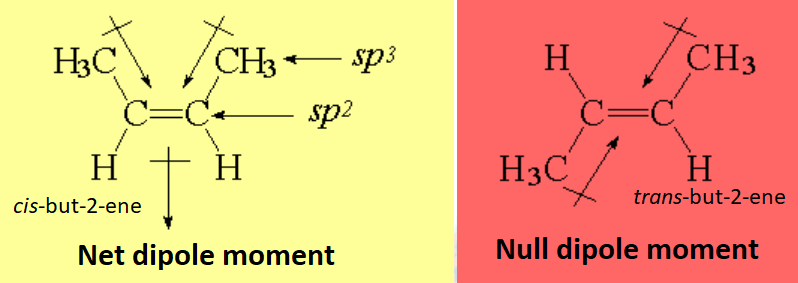
But polarity mainly depends on the E / Z stereochemistry of the olefin:
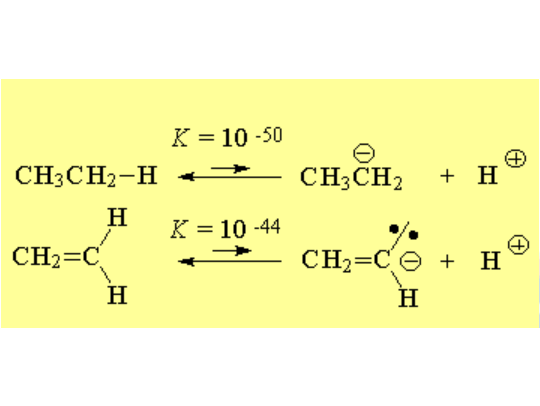
Alkenes cannot actually be catalogued as acids but, in relative terms, they are a million times more acidic than the corresponding alkanes.
This is proof of the higher electronegativity of the sp2 carbons relative to the sp3 ones.
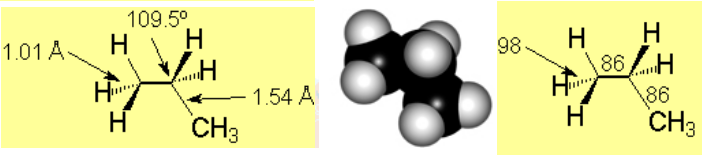 Angles, lenghts and
Angles, lenghts and 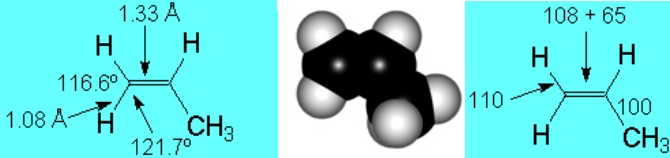 Angles, lenghts and
Angles, lenghts and