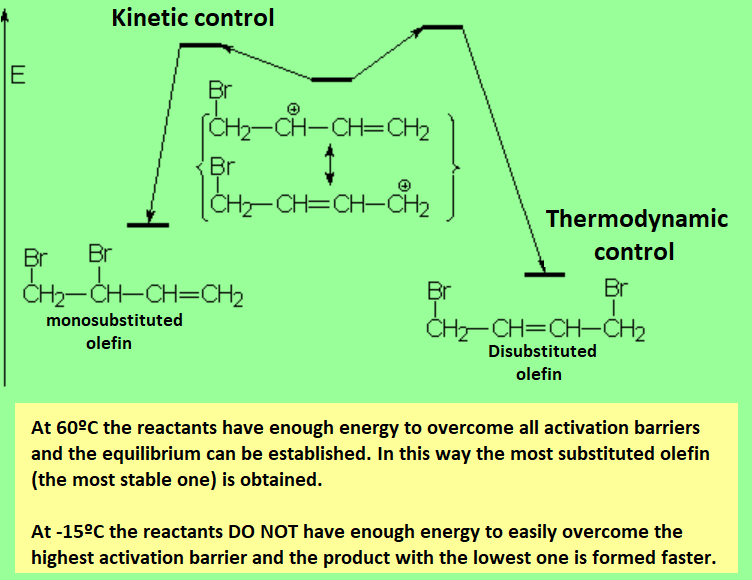
One of the two double C=C bonds attacks the bromine molecule, leading to an allylic carbocation, where the positive charge is delocalized between positions 1 and 3. That's why the reaction yields the normal (1,2) and conjugated (1,3) addition products.
If the electrophilic addition to a conjugated diene is carried out in thermodynamic control conditions (high temperatures and long reaction times), the ratio of conjugated addition increases, the 1,4 product being the major one in most cases.
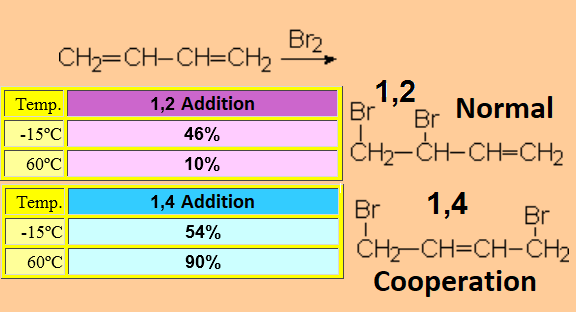 Isn't 1,4-dibromo-2-butene an unexpected product?
Isn't 1,4-dibromo-2-butene an unexpected product?