DIELS - ALDER CYCLOADDITION: REGIOCHEMISTRY AND STEREOCHEMISTRY
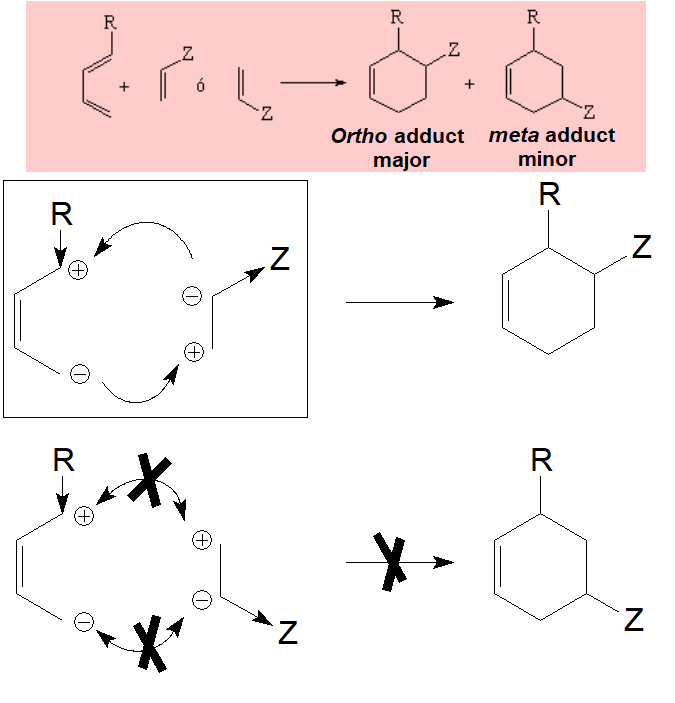
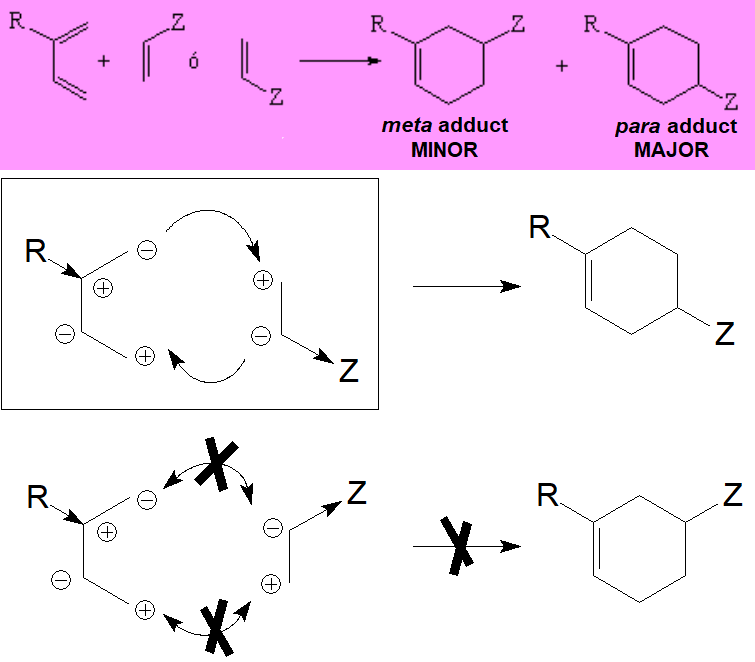
When diene and dienophile bear substituents two different regiochemical approaches are possible.
Yet, a well known experimental fact is that the "meta" adduct is NEVER the major component.
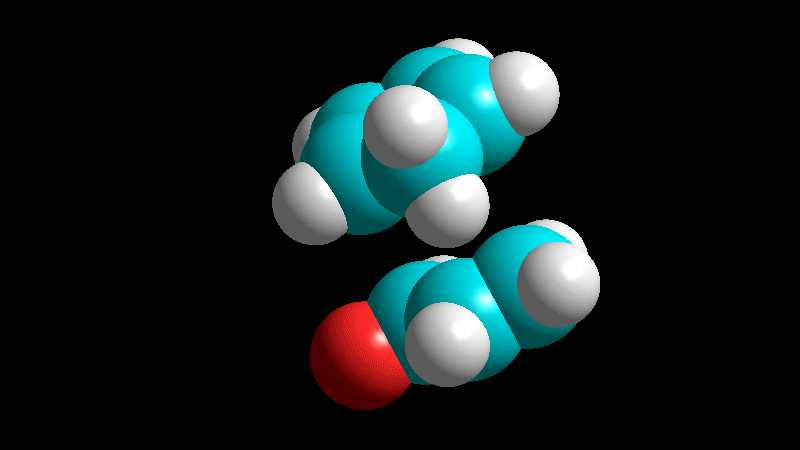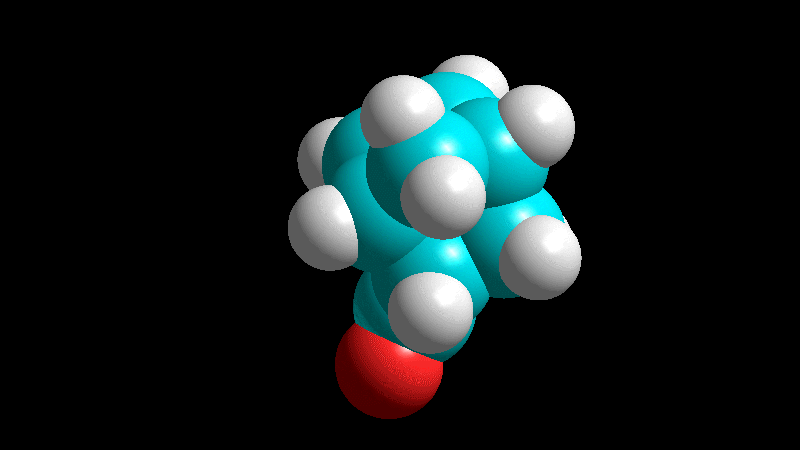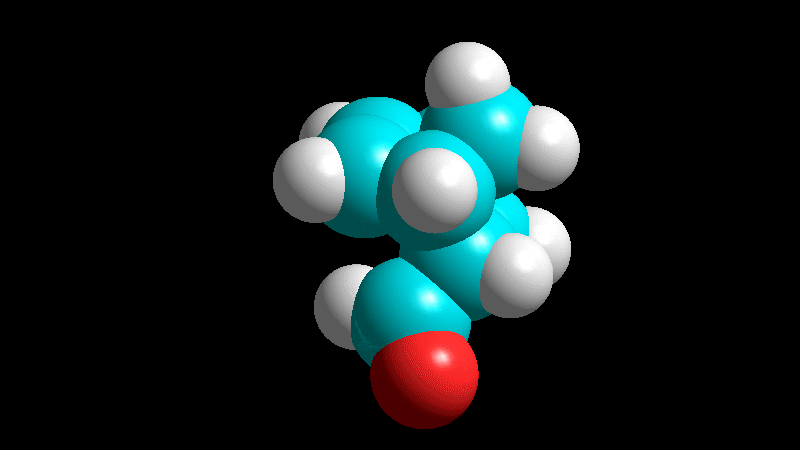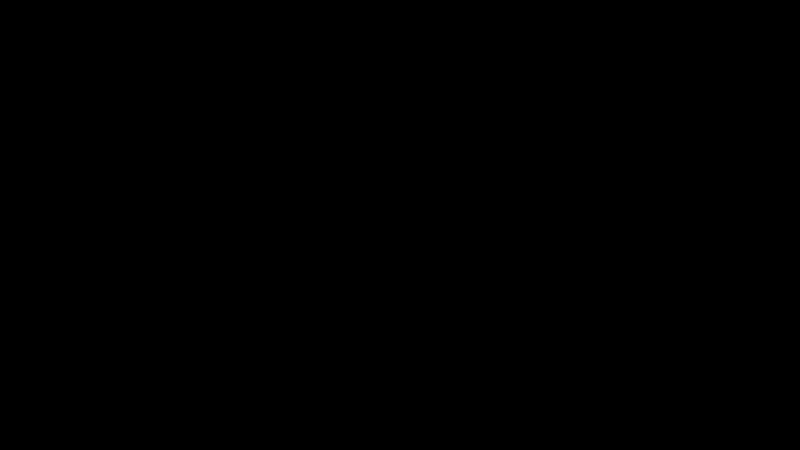
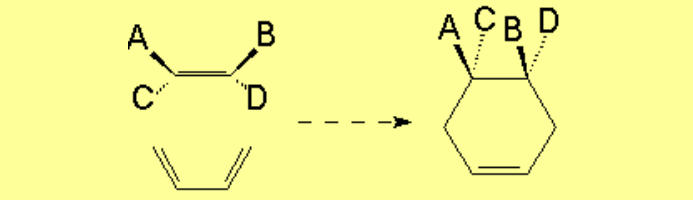
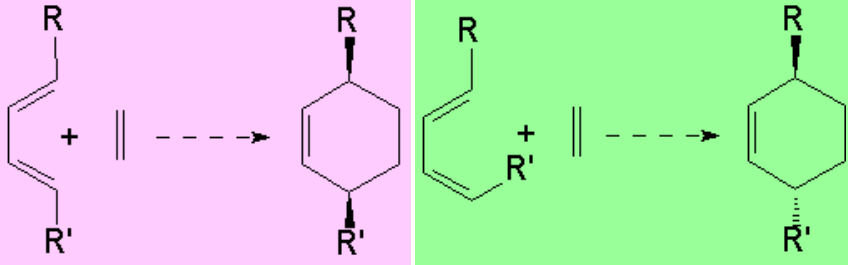
Relative to the dienophile, the addition is ALWAYS syn:
The cis groups in the olefin keep on being cis in the resulting cyclohexene.
The trans groups in the olefin keep on being trans in the resulting cyclohexene.
The addition is also syn concerning the diene:
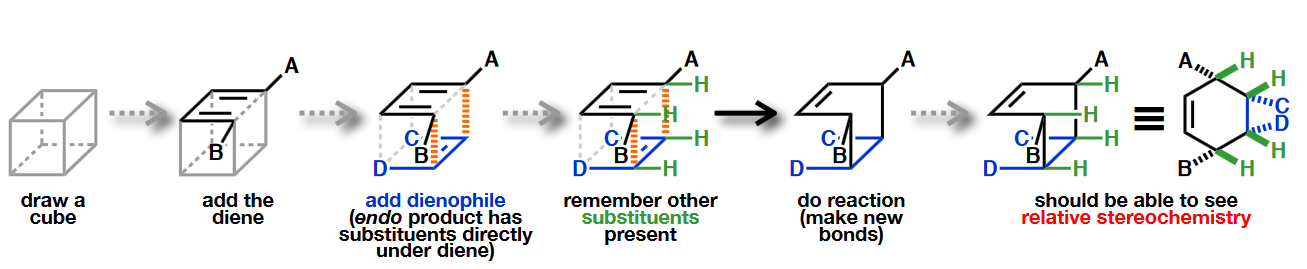
When the diene is cyclic and the dienophile is non-symmetric, there are two posible addition stereochemistries, so-called exo and endo.
The endo adduct usually predominates:
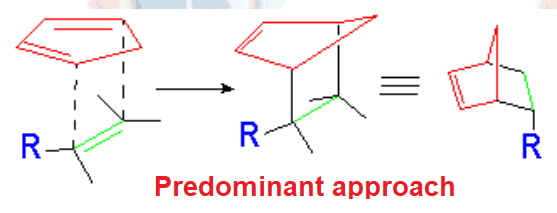 The dienophile adds with the R group poised to the inside (endo) of the diene system, leading to a bicycle (norbornene) with the R group in endo arrangement.
The dienophile adds with the R group poised to the inside (endo) of the diene system, leading to a bicycle (norbornene) with the R group in endo arrangement.
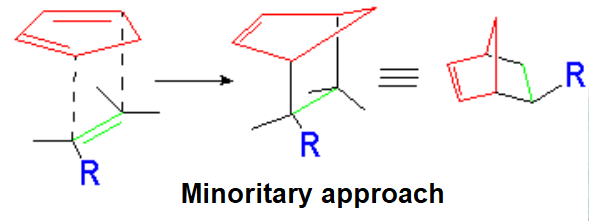 The dienophile adds with the R group pointing to the outside (exo) of the diene system, yielding a bicycle (norbornene) with the R group in exo arrangement.
The dienophile adds with the R group pointing to the outside (exo) of the diene system, yielding a bicycle (norbornene) with the R group in exo arrangement.
IMPORTANT: In all cases, if the final adducts end up with new stereocenters and the starting materials are not enantiomerically pure, the final products must be RACEMIC.
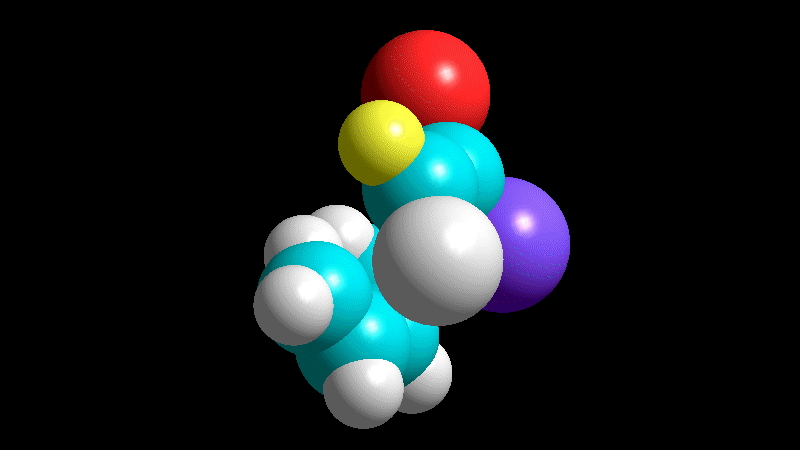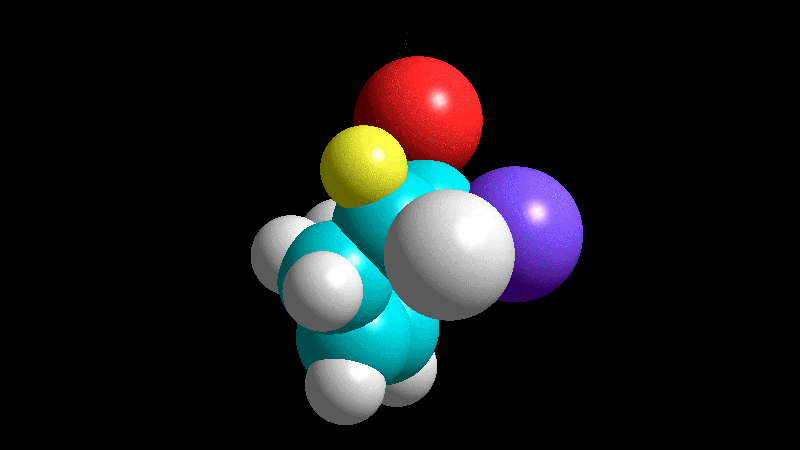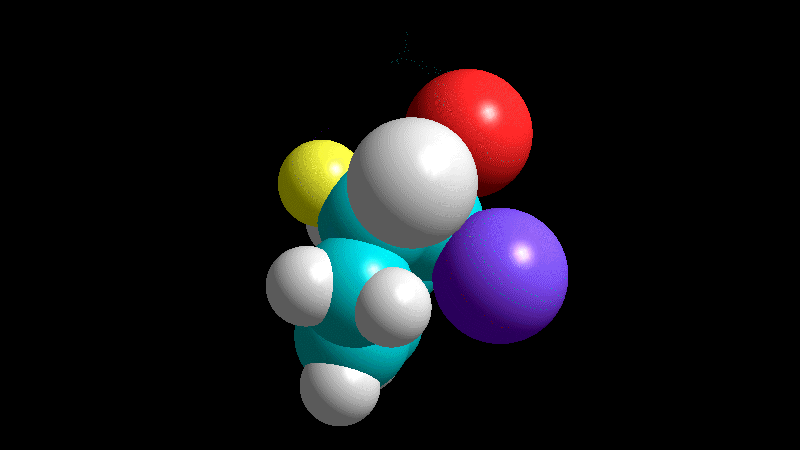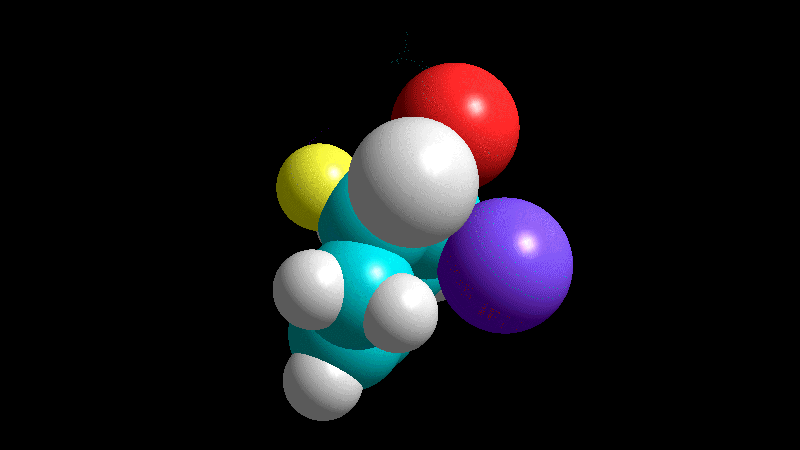

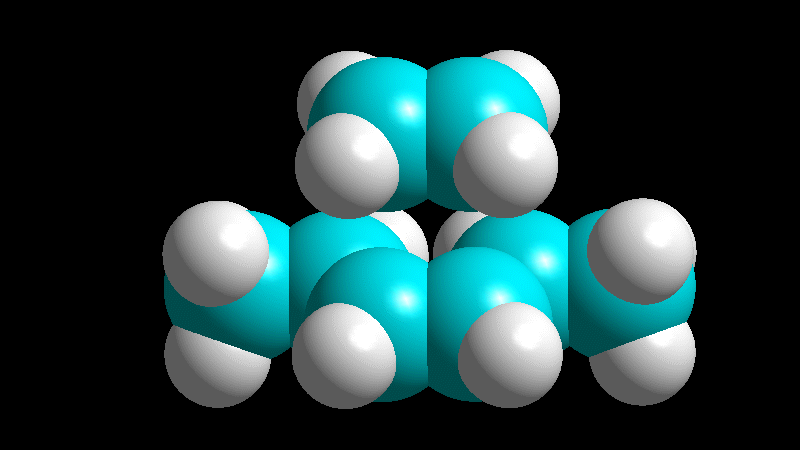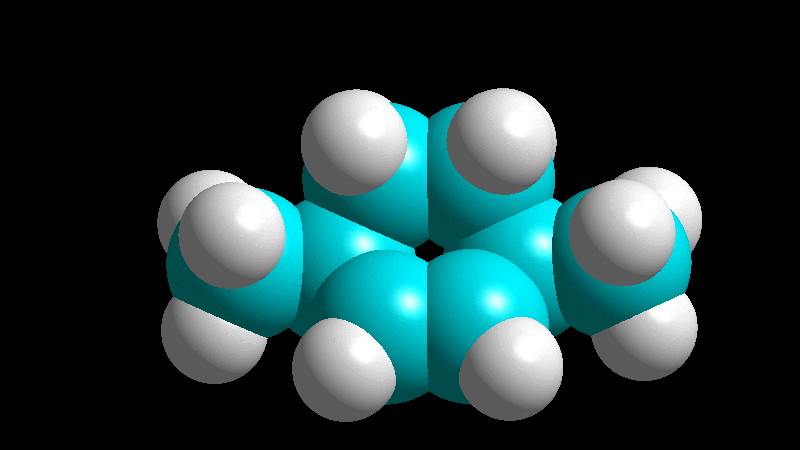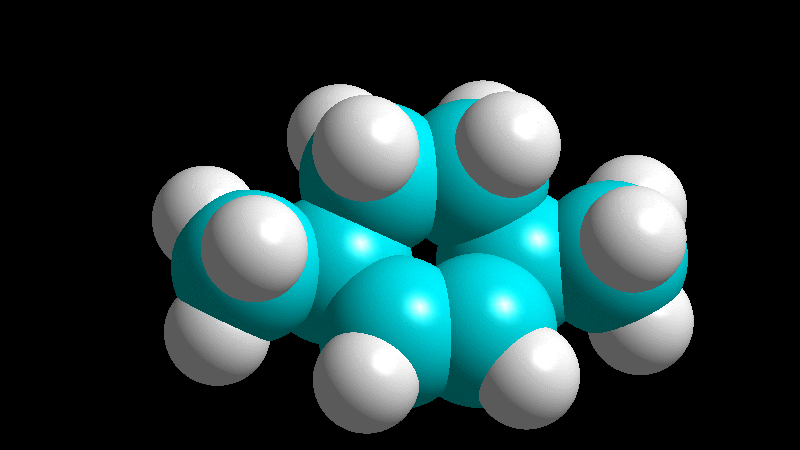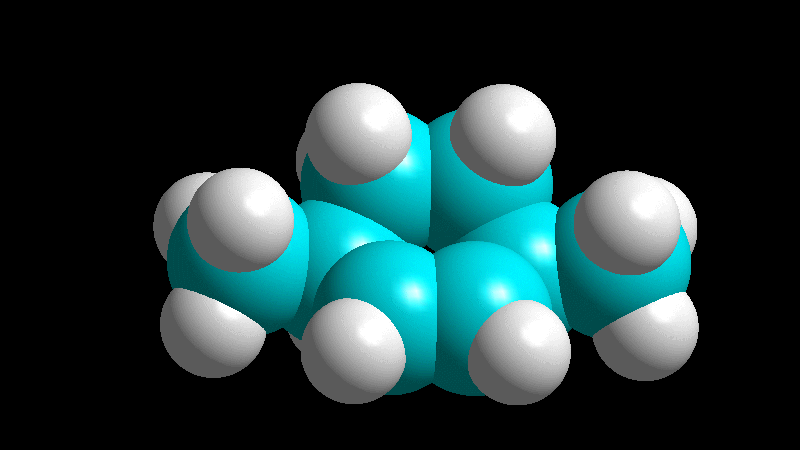
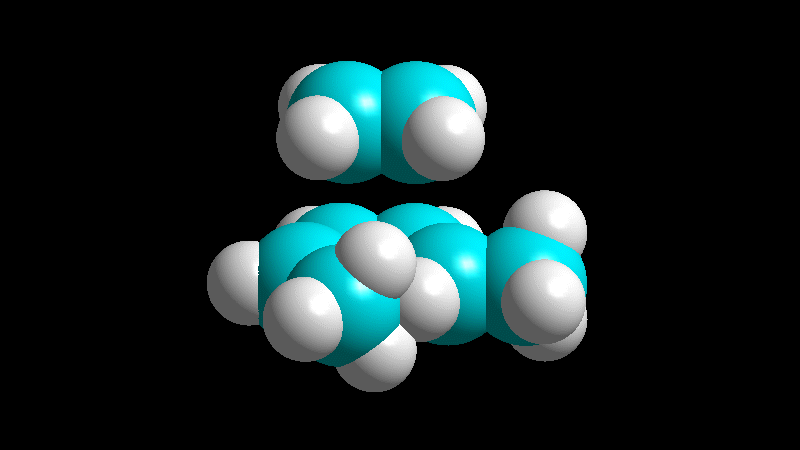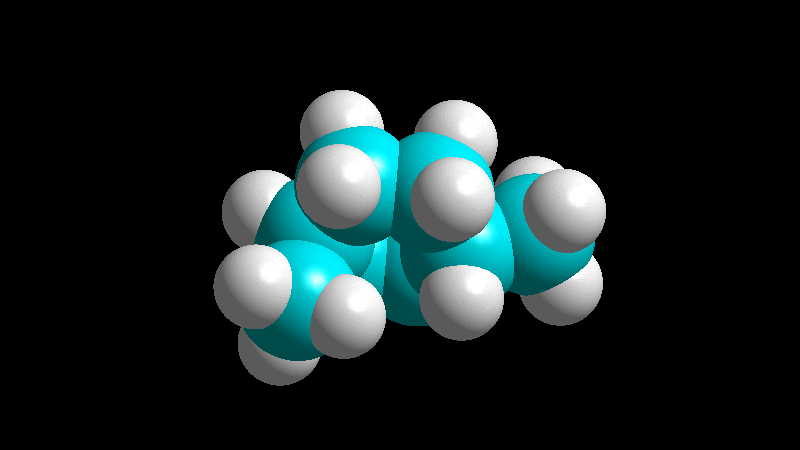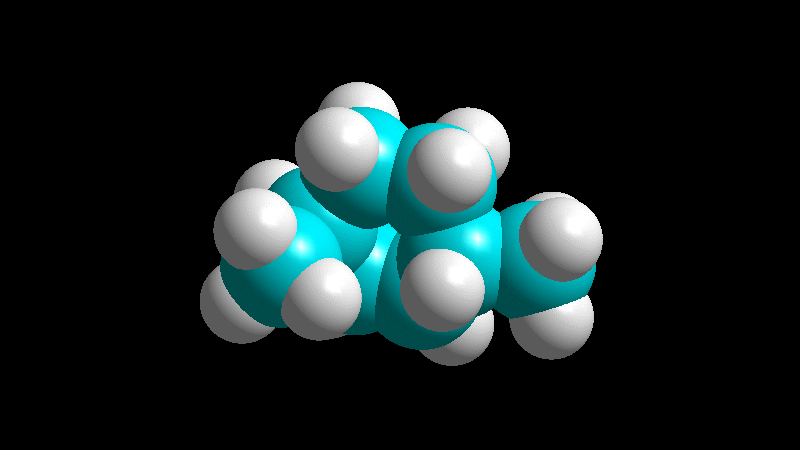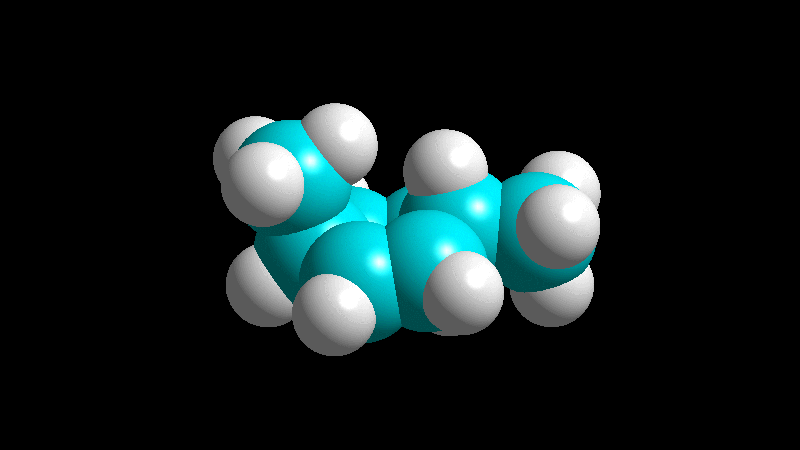
 The dienophile adds with the R group poised to the inside (endo) of the diene system, leading to a bicycle (norbornene) with the R group in endo arrangement.
The dienophile adds with the R group poised to the inside (endo) of the diene system, leading to a bicycle (norbornene) with the R group in endo arrangement.
 The dienophile adds with the R group pointing to the outside (exo) of the diene system, yielding a bicycle (norbornene) with the R group in exo arrangement.
The dienophile adds with the R group pointing to the outside (exo) of the diene system, yielding a bicycle (norbornene) with the R group in exo arrangement.