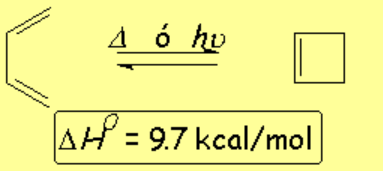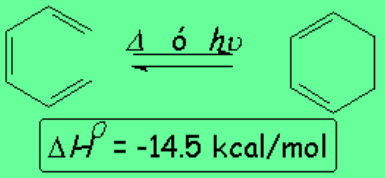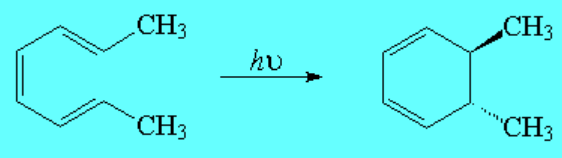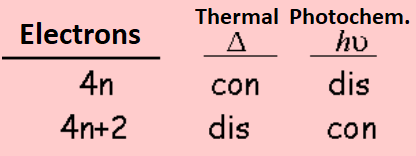Polyenes react within themselves, intramolecularly, clipping their ends and forming rings, either with temperature or light.
These reactions are named "pericyclic" because they proceed through a cyclic, closed transition state.
1,3-Butadiene cycles yielding cyclobutene.
The reaction is possible although the tension of the four-membered product makes it thermodynamically unfavorable.
1,3,5-Hexatriene produces a cyclohexadiene whose ring is much less tensioned than in the example above.
In this case the reaction is exothermic and thus thermodynamically favored.
However, the reaction is not spontaneous because the interaction of both ends of the cyclohexatriene system requires a highly ordered transition state (entropy demanding) and hence a sizable activation energy.
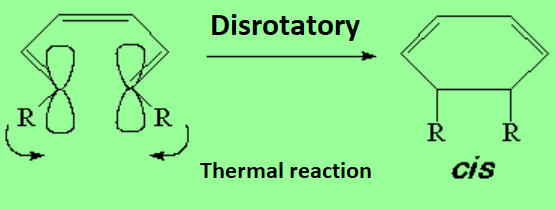
Amazingly, the stereochemistry of the products depends on whether the reaction is performed with just temperature or shining a light on it.
Look at the following examples: Aren't they surprising enough?
How can one explain the different outcome?
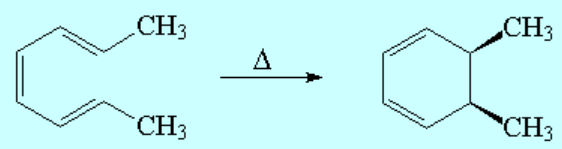
(E,Z,E)-2,4,6-Octatriene thermically cycles to exclusively yield cis-5,6-dimethyl-1,3-cyclohexadiene.
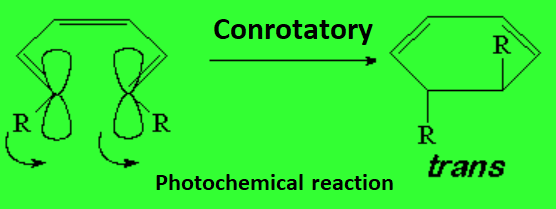
In contrast, to our astonishment, the same molecule at room temperature, but shining a light over it of a given wavelength leads to the trans isomer as the sole product.
With a simple model we can realize the twisting movement that both ends of the triene system must accomplish in order to form the new sigma bond. That movement must be undoubtedly different when the reaction is carried out under temperature or light because of the distinct stereochemical outcome.
The cis stereochemistry (thermal reaction) is attained if, and only if, the twisting movements at both ends of the triene are clockwise and counterclockwise respectively, i.e. the opposite.
That's why this way of ring closing is called disrotatory.
In order to obtain the trans stereochemistry (photochemical reaction), the twisting at both ends of the triene must be either clockwise or counterclockwise, i.e. the same.
Therefore, the name conrotatory makes sense for this process.
This puzzling outcome has to be explained someway. How can we rationalize the different outcome depending on such apparently tiny difference: temperature or light?
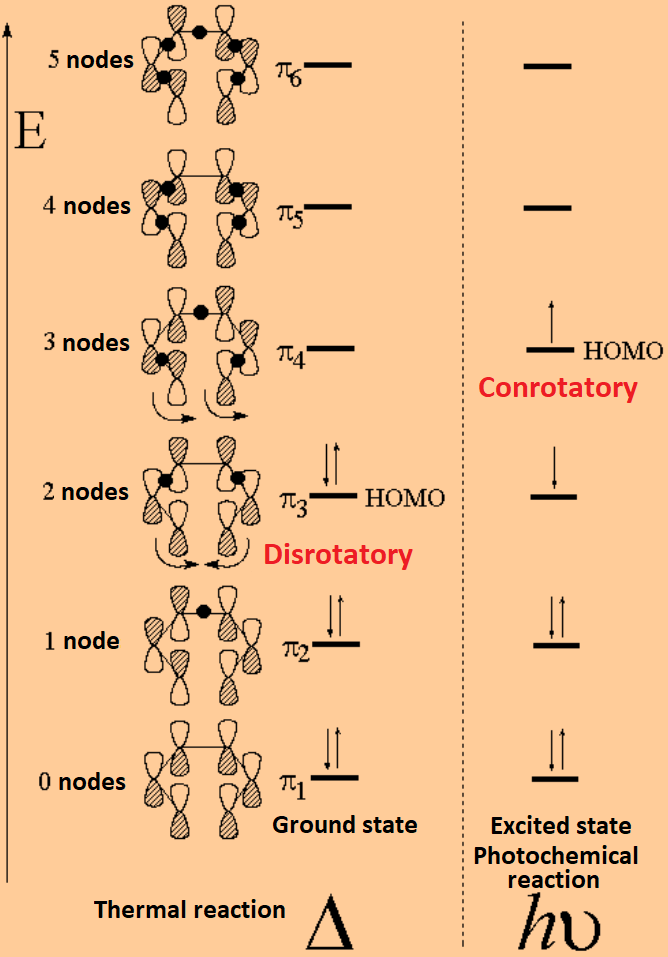
Let's have a look at the "pi" molecular orbitals which are key in this reaction.
The combination of six carbon atoms gives rise to six "pi" molecular orbitals of increasing antibonding character. Every carbon contributes with one electron.
It's easily seen that the symmetry at both ends of the reacting HOMO (Highest Occupied Molecular Orbital) is different in the ground or excited states.
This fact can be generalized for a system of 4n or 4n+2 electrons. Woodward and Hoffman were awarded the Nobel Prize of Chemistry for the clever development of this simple idea, that is summarized in the following Table:
By carefully drawing the MOs of a polyenic system and by properly considering the symmetry at both ends of the HOMO, the allowed twisting mode can be easily predicted and the resulting stereochemistry as well.
Awesome!!!
Robert Burns Woodward (1917-1979): North American Chemist. He performed lots of total synthesis of complex organic compounds like Quinine, Cholesterol, Cortisone and Stricnine among many others. He studied the spatial arrangement of antibiotics and carried out useful modifications in order to improve their properties. He accomplished the synthesis of Chlorophil. Nobel Prize of Chemistry in 1965.
Roald Hoffmann (1937- ): North American Chemist, Polish in origin. Together with Woodward, he formulated the rules for an important family of chemical reactions, based on molecular orbital symmetry. Nobel Prize of Chemistry shared with Fukui Kenishi in 1981.