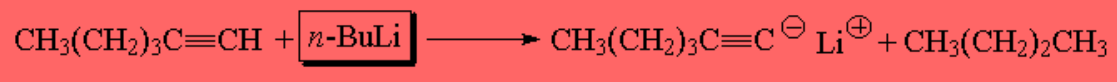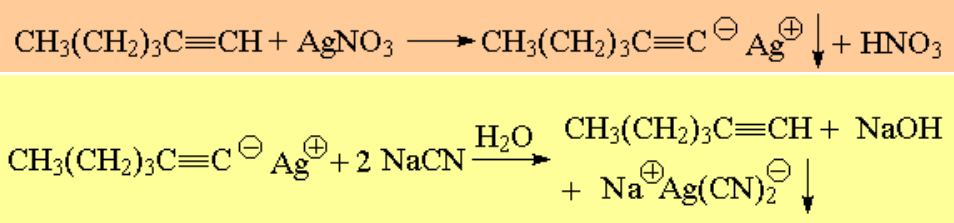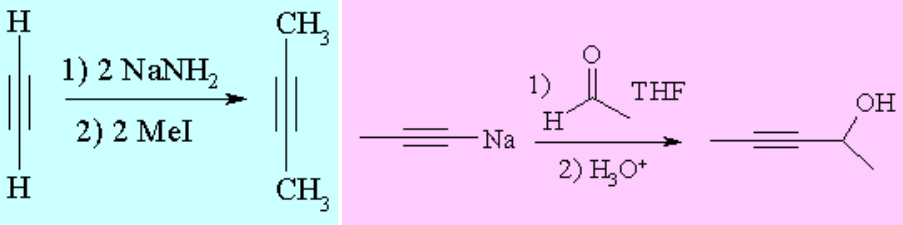PHYSICAL AND BOND PROPERTIES OF ALKYNES
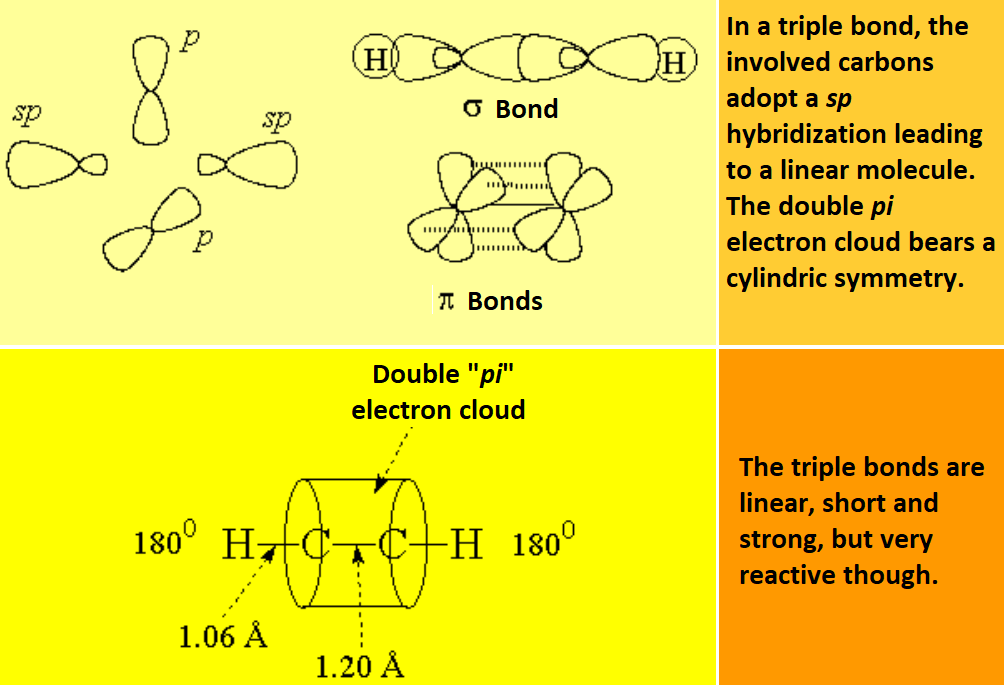
A triple bond is constituted by the frontal overlap of two sp hybrid orbitals, leading to a "sigma" bond, and the lateral overlap of four p non-hybrid orbitals, yielding two"pi" bonds:
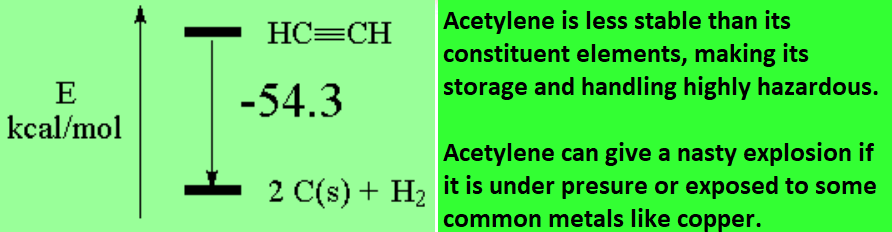
Compare the bond energies (homolytic cleavage):
The alkynes are similar in physical properties to the corresponding alkanes and alkenes. A triple bond makes little difference.

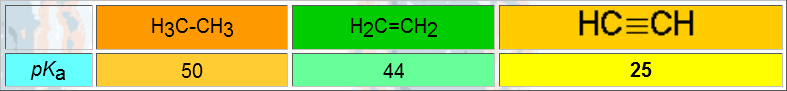
VERY IMPORTANT: Acetylene and terminal alkynes are relatively acidic.
When one forms an anion from a terminal alkyne, the negative charge dwells in a hybrid sp orbital. The larger the s character of a hybrid orbital, the closer and the more attracted the electrons are by the nucleus and hence, the more stable they are and the less basic the anion is.
That's why the conjugated acid, i.e. the alkyne, is much more acidic than an alkane.
Due to the relatively large differences in pKa, the butyl anion (derived from butyllithium) is able to detach the proton from a terminal alkyne:
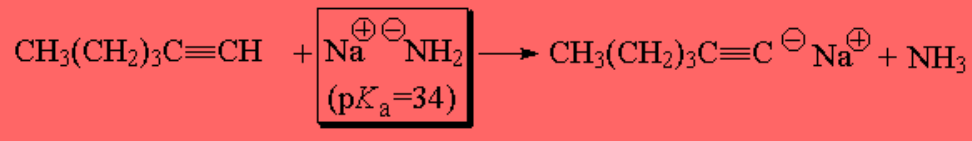
Sodium amide is able too to extract the terminal alkyne's proton:
Terminal alkynes render insoluble salts with Ag+ and Cu+ cations. The alkyne can be regenerated by the action of cyanide:
The so-called alkynides, the anions obtained by the action of a strong base over a terminal alkyne, are excellent nucleophiles that can be used in either nucleophilic substitutions or additions to build new C-C bonds.