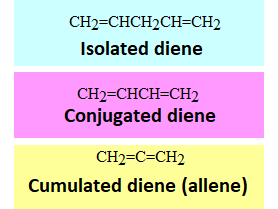
PHYSICAL AND BOND PROPERTIES OF DIENES
Isolated dienes do not show any different properties to those of plain alkenes.
However, the cumulated and conjugated dienes do.
Cumulated Dienes (Allenes)
In order to explain the structure of an allene (cumulated diene), the central carbon must be considered as sp hybridized, NOT sp2.
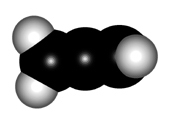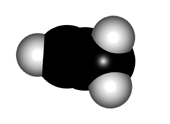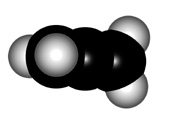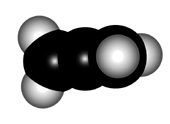 The higher "s" character of the hybrid "sp" orbitals of the central carbon makes the double C=C bonds shorter than those of plain alkenes.
The higher "s" character of the hybrid "sp" orbitals of the central carbon makes the double C=C bonds shorter than those of plain alkenes.
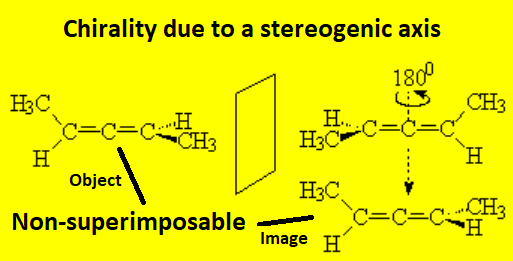 2,3-Pentadiene has two enantiomeric structures. Each of them displays optical rotation.
2,3-Pentadiene has two enantiomeric structures. Each of them displays optical rotation.
Allenes can be chiral.
Although allenes do not strictly possess any stereocenter, those with different substituents at both ends of the cumulated doble C=C bonds happen to bear a chiral axis that makes object and mirror image be non-superimposable.
In order to assign the R/S configuration to a chiral allene, the four substituents should be appropriately numbered. Start with the carbon in the front and then with that in the back of the allene following the usual prelation rules.
Then draw a tetrahedron as it is shown in the Figure, place the least important substituent (4) to the back and look at the clockwise (R) or anti-clockwise (S) turn necessary to go from 1 to 2 and 3.
The central bond of a conjugated diene has a partial double-bond character.
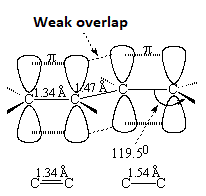 The length of the central bond is intermediate between true simple C-C or double C=C bonds.
The length of the central bond is intermediate between true simple C-C or double C=C bonds.
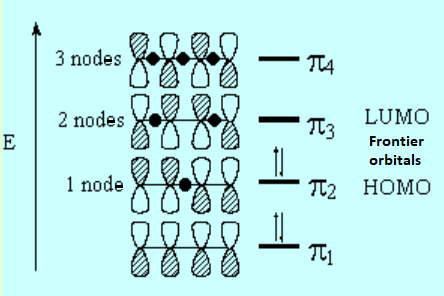 The lateral overlap of the four atomic "p" orbitals produces four molecular "pi" orbitals. The "pi1" orbital delocalizes some electronic density along the four atoms involved in the conjugation. However, the "pi2" orbital doesn't. This explains the partial double bond character of the central bond.
The lateral overlap of the four atomic "p" orbitals produces four molecular "pi" orbitals. The "pi1" orbital delocalizes some electronic density along the four atoms involved in the conjugation. However, the "pi2" orbital doesn't. This explains the partial double bond character of the central bond.
The small contribution of the charge-separated resonant forms also explain the partial double bond nature of the central bond.
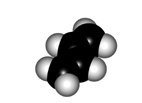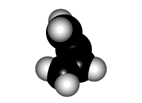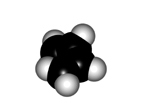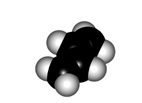
The twisting of the central bond of a conjugated diene displays different features to those of a true single C-C bond of alkanes:
In order to make the "p" orbitals overlap in the diene framework, the whole system has to be planar.
Yet, the central bond could be twisted giving rise to two conformers named as s-trans (more stable) and s-cis.
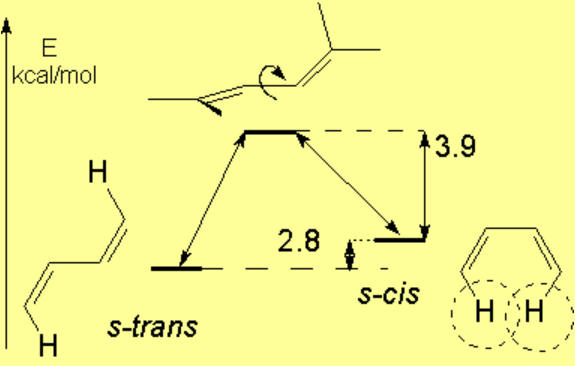 This bond twisting is more costly (6.7 kcal/mol) than in an alkane, due to the fact that in the TS the orbital overlapping "pi1" is distroyed because the planes of the double C=C bonds are perpendicular.
This bond twisting is more costly (6.7 kcal/mol) than in an alkane, due to the fact that in the TS the orbital overlapping "pi1" is distroyed because the planes of the double C=C bonds are perpendicular.
BLUE = Transition state (TS)
RED = Steric hindrance attained in the s-cis conformation.
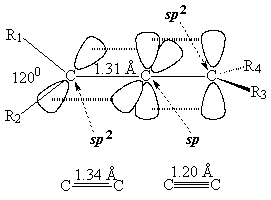
 The higher "s" character of the hybrid "sp" orbitals of the central carbon makes the double C=C bonds shorter than those of plain alkenes.
The higher "s" character of the hybrid "sp" orbitals of the central carbon makes the double C=C bonds shorter than those of plain alkenes. 2,3-Pentadiene has two enantiomeric structures. Each of them displays optical rotation.
2,3-Pentadiene has two enantiomeric structures. Each of them displays optical rotation.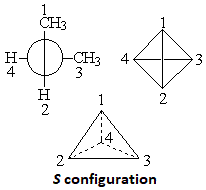
 The length of the central bond is intermediate between true simple C-C or double C=C bonds.
The length of the central bond is intermediate between true simple C-C or double C=C bonds. The lateral overlap of the four atomic "p" orbitals produces four molecular "pi" orbitals. The "pi1" orbital delocalizes some electronic density along the four atoms involved in the conjugation. However, the "pi2" orbital doesn't. This explains the partial double bond character of the central bond.
The lateral overlap of the four atomic "p" orbitals produces four molecular "pi" orbitals. The "pi1" orbital delocalizes some electronic density along the four atoms involved in the conjugation. However, the "pi2" orbital doesn't. This explains the partial double bond character of the central bond.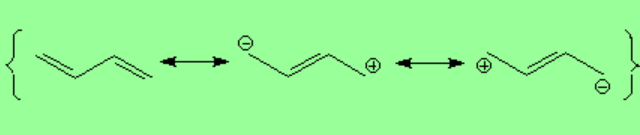
 This bond twisting is more costly (6.7 kcal/mol) than in an alkane, due to the fact that in the TS the orbital overlapping "pi1" is distroyed because the planes of the double C=C bonds are perpendicular.
This bond twisting is more costly (6.7 kcal/mol) than in an alkane, due to the fact that in the TS the orbital overlapping "pi1" is distroyed because the planes of the double C=C bonds are perpendicular.