SEAr IN POLYCYCLIC AROMATIC COMPOUNDS
Polycyclic aromatic compounds undergo the same SEAr that benzene does.
But there are some new details to be aware of.
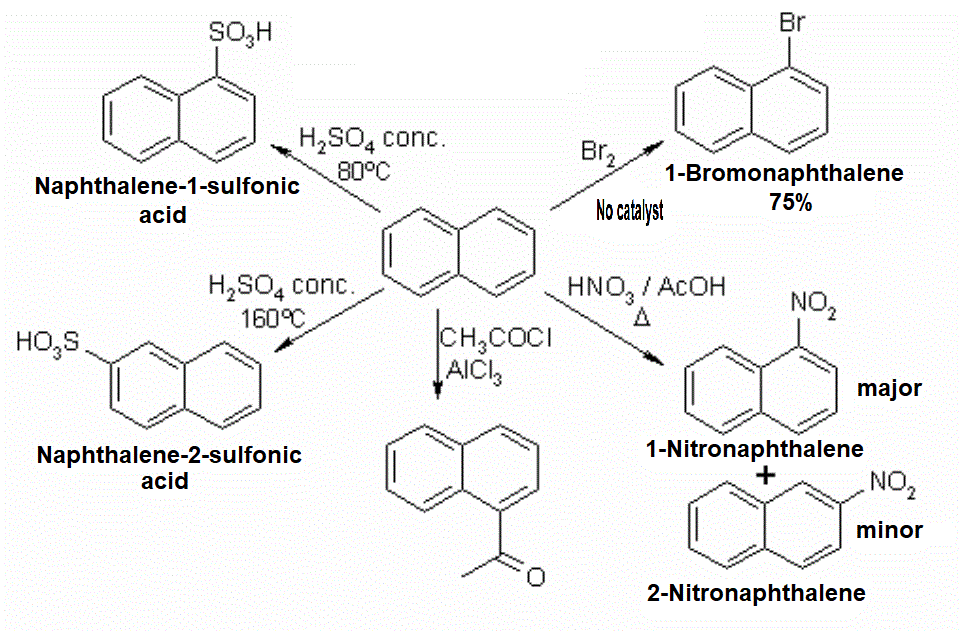
Naphthalene is more reactive than benzene.
For one, bromination does not need catalysis and nitration can be performed with acetic acid, much less stronger than sulfuric acid.
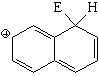
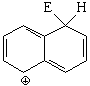
Position 1 is the most reactive in naphthalene.
It can be seen that, exception made of high-temperature sulfonation, the attacked position is always 1 (alpha).
The account of the possible reaction intermediates by resonance forms easily allows us to understand that reactivity trend.
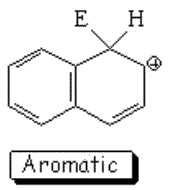
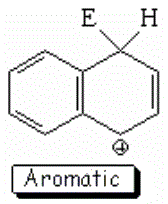
SEAr at position 1 (alpha) of naphthalene
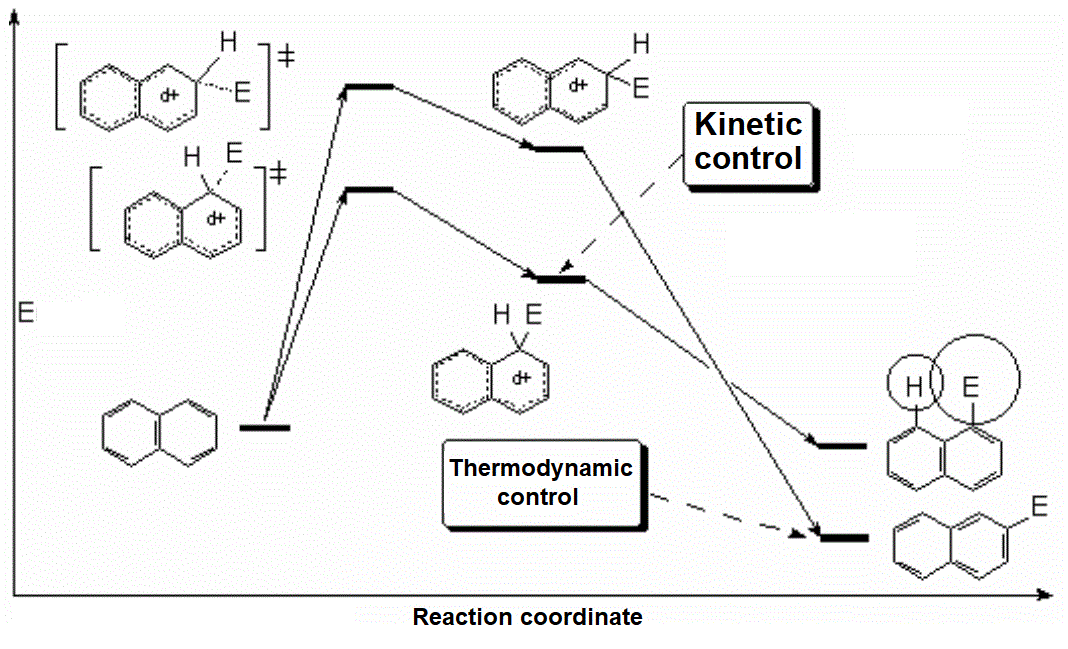
The resonance hybrid of this intermediate is quite aromatic and hence less unstable because it has two resonance forms that keep complete aromaticity of at least one ring.
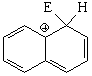
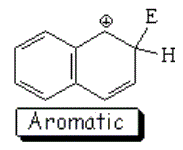
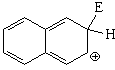
SEAr at position 2 (beta) of naphthalene
The resonance hybrid of this intermediate is less aromatic than the previous one and is thus more unstable because it has only one resonance form where the aromaticity of one ring is kept.
The paradox is that the substitution at position 1 (alpha) yields the most sterically congested product and therefore the least thermodynamically stable one.
Yet, the pathway leading to the 1 (alpha) substitution is the lowest in energy because the corresponding intermediate (see above) is less unstable.
Therefore, the substitution product at position 1 (alpha) occurs at a fast pace (kinetic control). However, in the case of the reversible sulfonation, if one lets the reaction proceed during a longer time and at a high temperature, the major product is the 2-substituted one (beta; thermodynamic control).
Do you catch it?
Polycyclic aromatic compounds can afford the permanent loss of some aromaticity in some reactions.
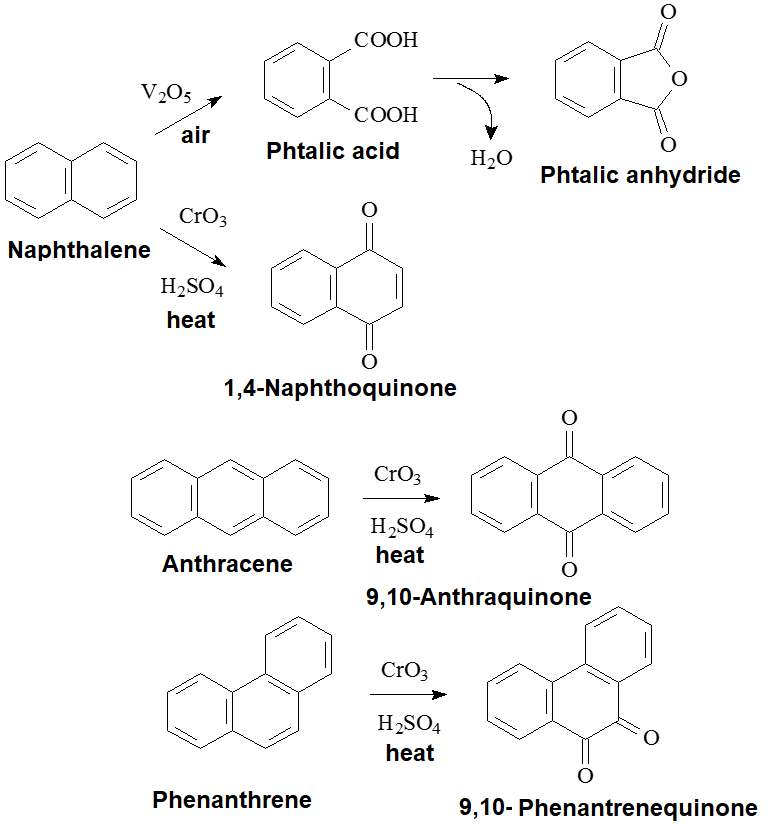
OXIDATION
The strong oxidation of naphthalene leads to the cleavage of one ring.
Its smooth oxidation gives a naphthoquinone instead.
Anthracene oxidation is performed in the central ring where the aromaticity was low from the beginning.
Phenanthrene also oxidizes in the central ring by the same token.
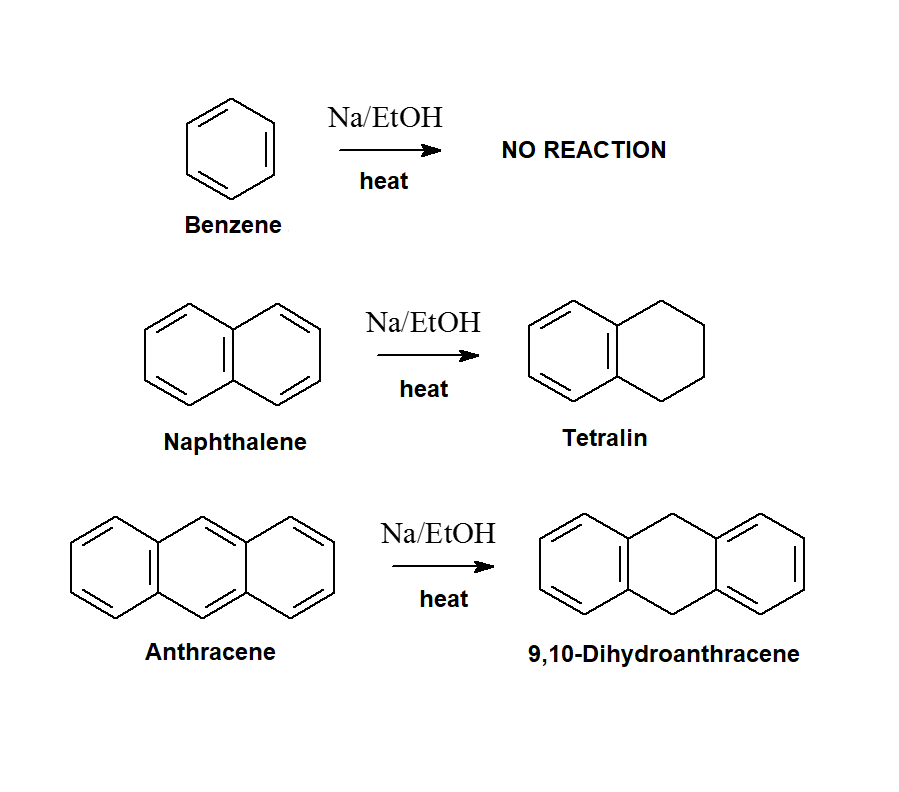
REDUCTION
In the same conditions in which benzene would have never been oxidized, naphthalene does by accepting four electrons from sodium.
The resulting tetrahydronaphthalene still preserves one ring's aromaticity.
Anthracene accepts two electrons in the same conditions, the central ring is thus reduced and the aromaticity of two rings is preserved.