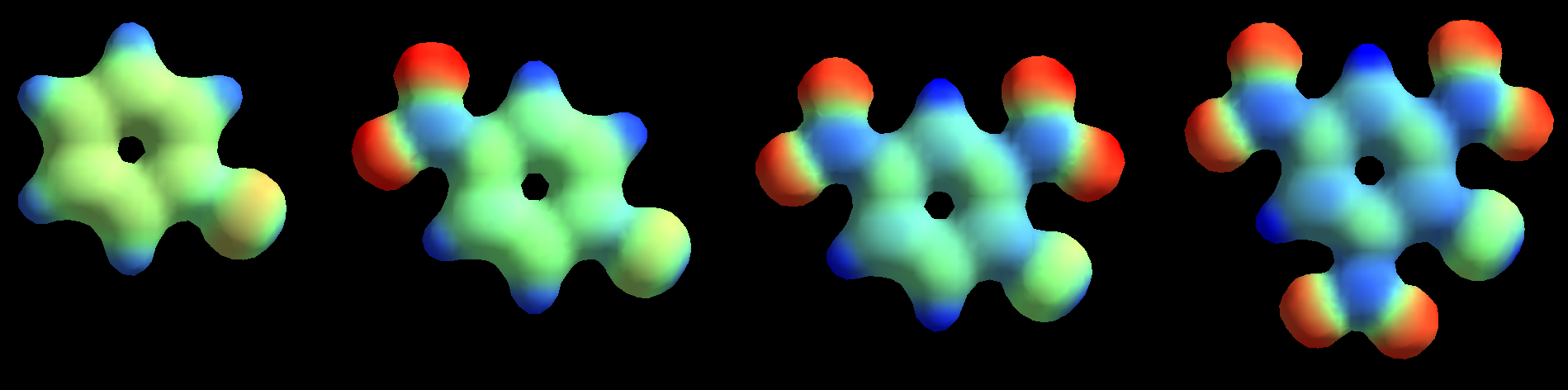NUCLEOPHILIC AROMATIC SUBSTITUTION SNAr
Aryl halides can undergo nucleophilic substitution reactions (SNAr) but in a very different manner as compared to how alkyl halides do it.
The "normal" reaction of an aromatic compound is the SEAr because the ring is electron rich. However, should the aromatic compound bear one or more electron-attracting substituents, the nucleophilic substitution (SNAr) can be carried out.
Chlorobenzene
1-Chloro-4-nitrobenzene
1-Chloro-2,4-dinitrobenzene
1-Chloro-2,4,6-trinitrobenzene
The presence of an increasing number of strong electron-withdrawing nitro groups, makes the ring be increasingly electron defficient (the blue color increases).
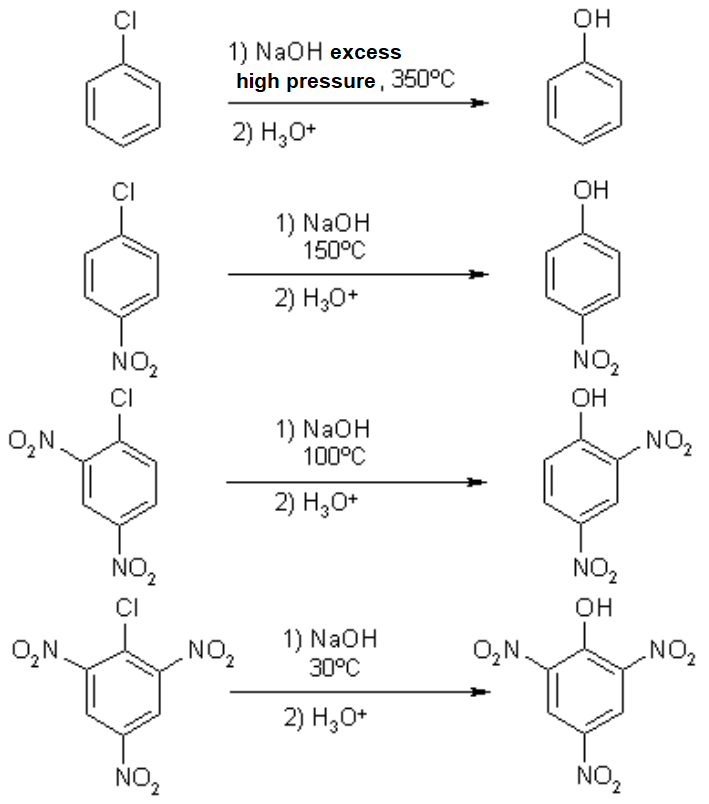
In this situation, a good leaving group, like chlorine, can be displaced by a strong nucleophile, like hydroxide:
Chlorobenzene reacts with hydroxide in very drastic conditions. In contrast, the same reaction on 2,4,6-trinitrochlorobenzene can be performed in much more smooth conditions, proving that a SNAr process is strongly facilitated if there are electron-withdrawing groups attached to the ring.
The nucleophilic attack by (HO-) comes from a perperdicular trajectory to the ring plane, just to where the "p" orbitals forming the "pi" cloud are pointing to.
The mechanism of the SNAr has nothing to do with the aliphatic substitution (either SN1 or SN2) on sp3 carbons.
Nevertheless, it's sure you will find a pronounced resemblance to the SEAr, the main difference being that in the SNAr a negative (instead of positive) charge is developed. This is reasonable because in the SNAr the attacking species (nucleophile) bears an electron excess.
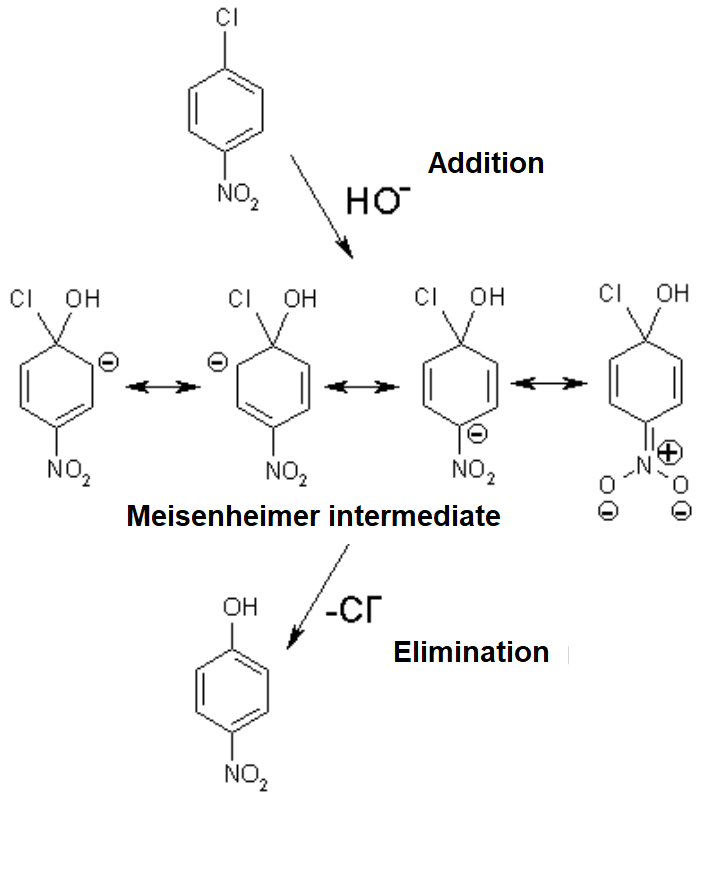
The electron defficiency displayed by the carbon bonded to the chlorine, increased by the resonance effect of the para-NO2, makes it easy for the hydroxide to attack it.
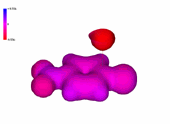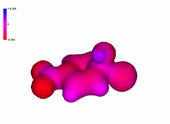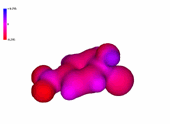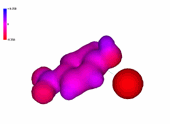
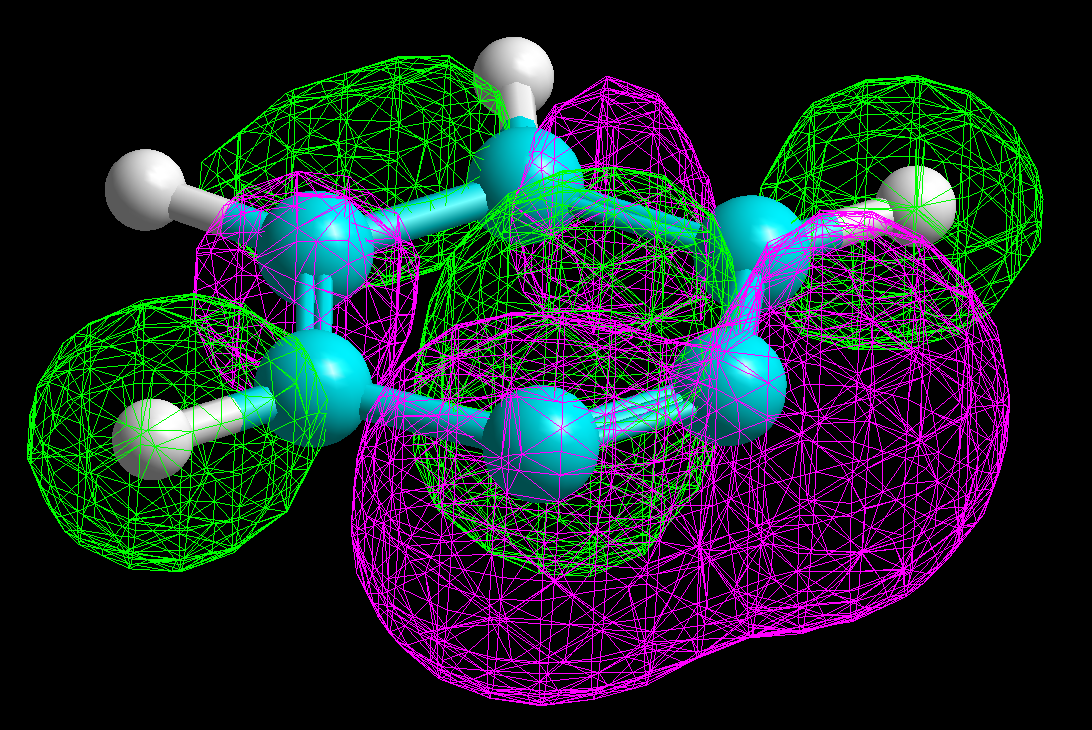
A negatively-charged intermediate is thus formed, named after its discoverer german chemist (Meisenheimer).
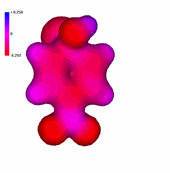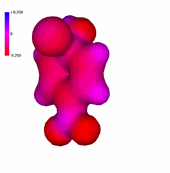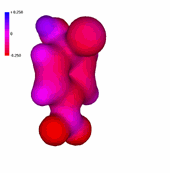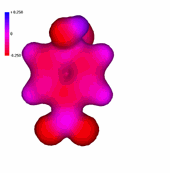
It can be seen in the molecular model how the negative charge concentrates in the ortho and para positions relative to the attack site.
The resonance forms help us to understand that.
The 4-nitro group assists in the stabilization of the developed negative charge.
Did you understand the mechanism?
If you did you must then be able to answer the following question:
Why 3-nitrochlorobenzene reacts less easily with hydroxide than its counterpart 4-nitrochlorobenzene?
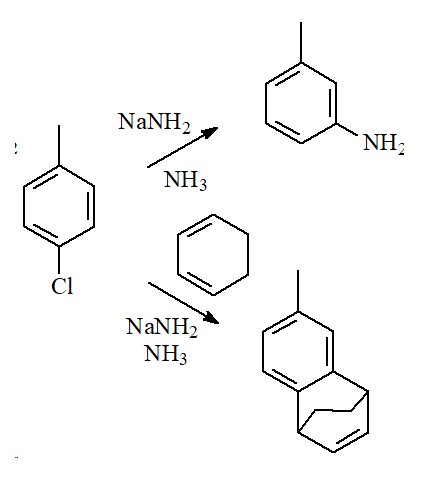
There is another mechanism that explains the following facts:
For example:
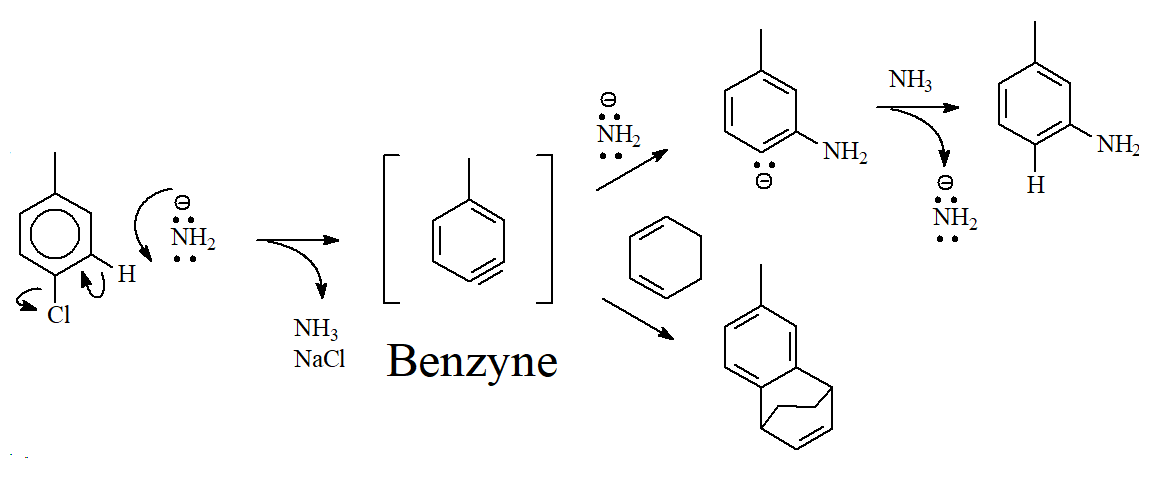
1) Toluene reacts with the strong base sodium amide and produces m-toluidine(m-methylaniline).
2) When the same reaction is carried out in the presence of a good diene, like cyclohexadiene, the Diels-Alder adduct is formed.
Benzyne must be involved because otherwise the formation of the Diels-Alder adduct cannot be understood:
The structure of benzyne is not explained as a conventional triple bond in which there are two regular "pi" clouds formed by the overlap of the corresponding "p" orbitals.
In the case of benzyne, one of the "pi" clouds is formed from the incomplete overlap of two vicinal sp2 orbitals sharing two electrons. The overlap is incomplete because the two sp2 orbitals are not parallel for obvious geometrical reasons.
Conventional triple bonds are reactive but very stable. Benzyne is very unstable and extremely reactive. That's why it has to be "captured" in situ with the diene.