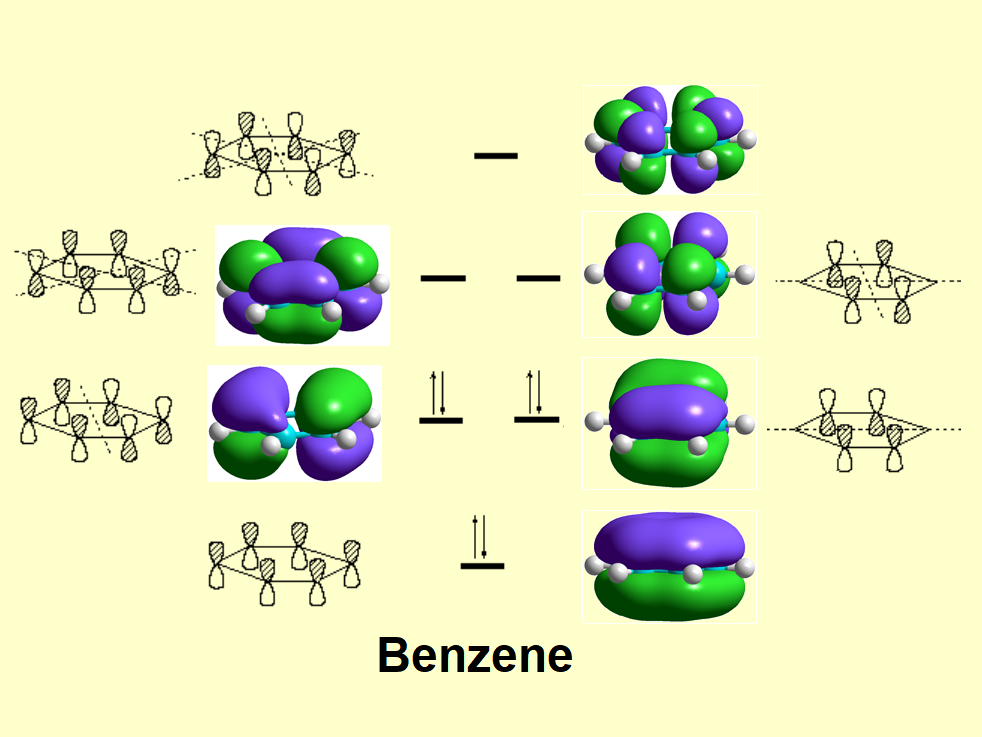
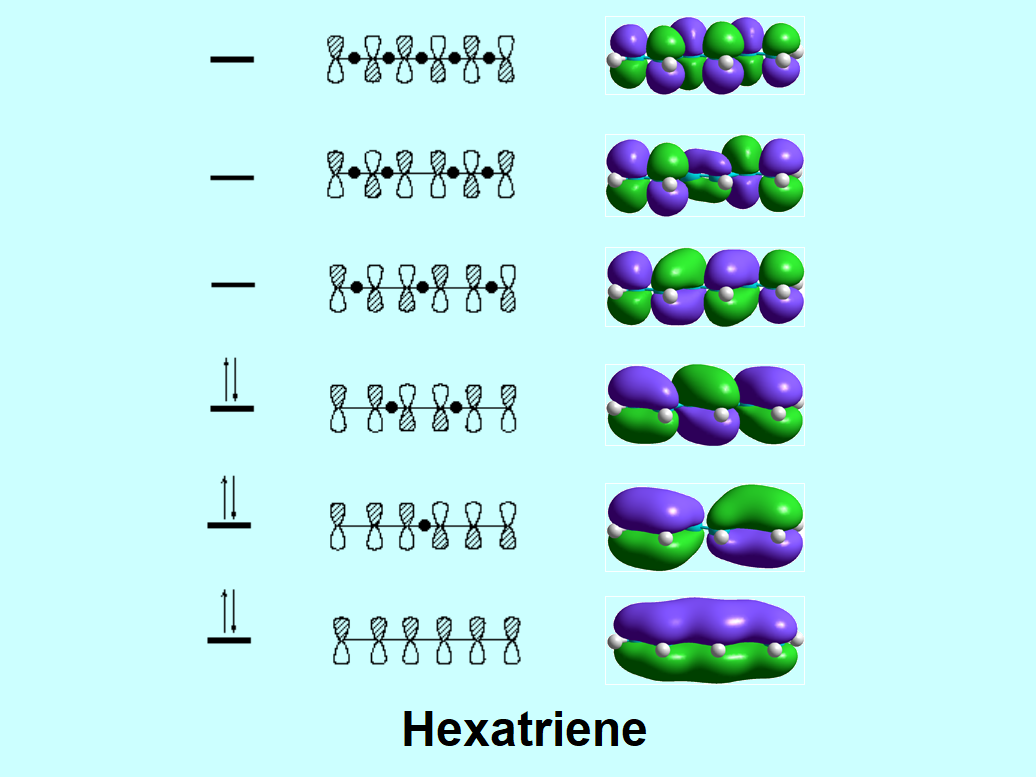
Let's compare benzene's and hexatriene's " pi " molecular orbitals. You should find a certain similarity: delocalization.
But you must realize an important difference: degeneration. Benzene has two pairs of degenerated orbitals, i.e. bearing the same energy. We already know that delocalization means stabilization. Yet, degeneration is also a synonym of added stability.
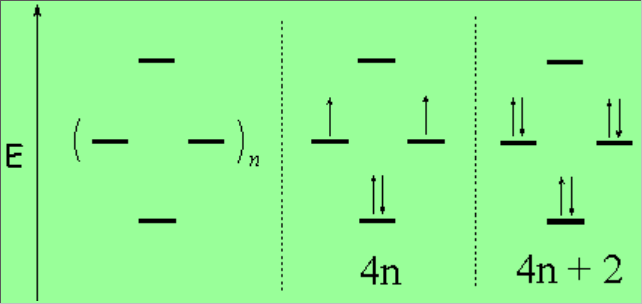
Hückel set forth rules for aromaticity and antiaromaticity:
A system of pi bonds is aromatic if:
1) fully conjugated
2) cyclic
3) planar
4) contains 4n + 2 pi electrons.
IMPORTANT:
A set of 4n electrons in the same conditions is antiaromatic and highly unstable.
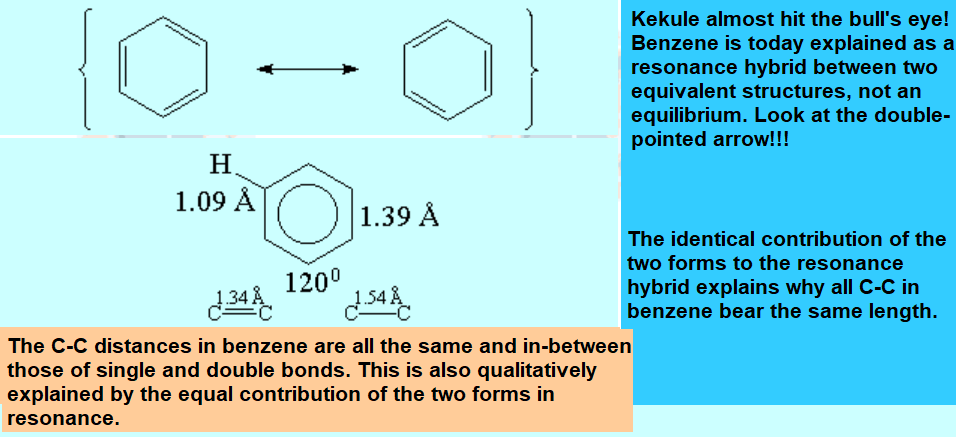
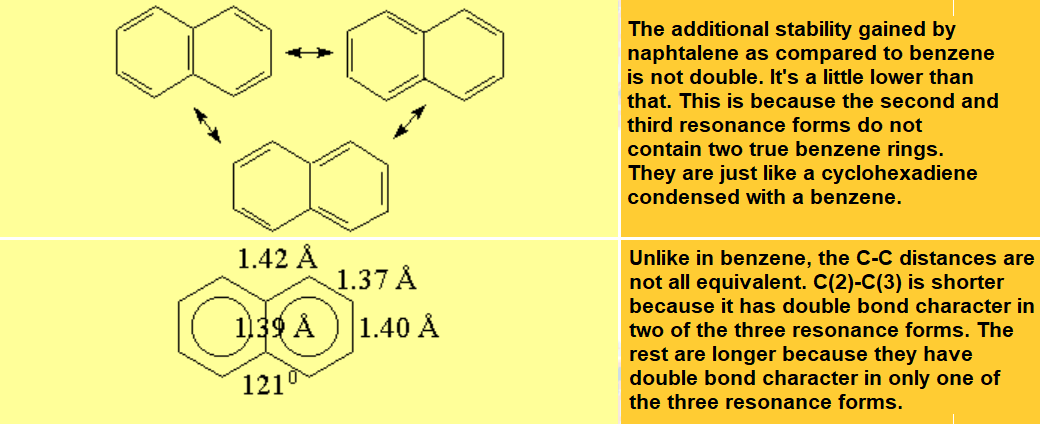
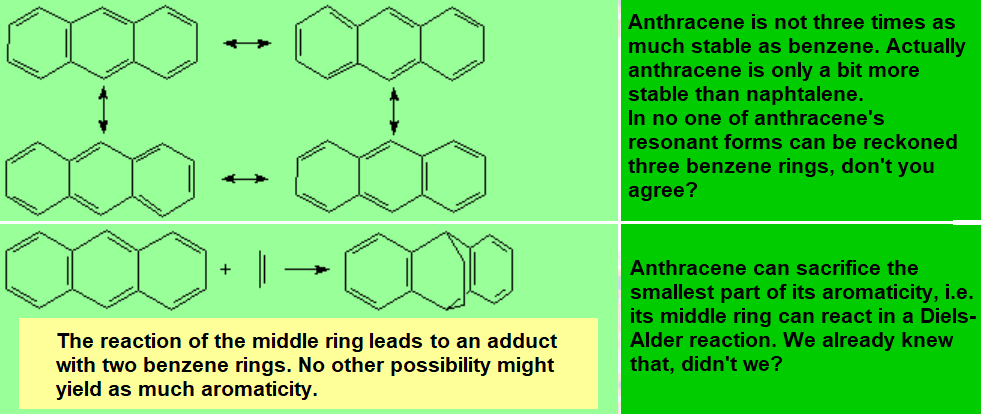
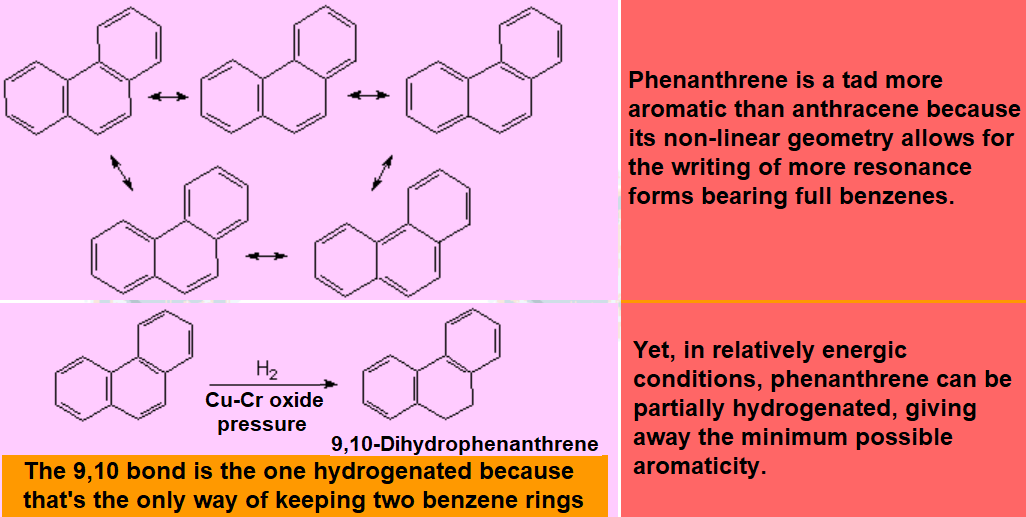
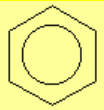
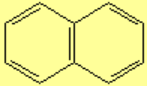
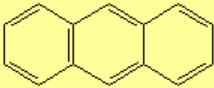
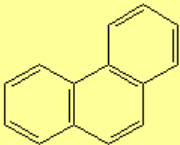
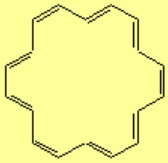
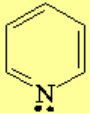
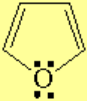
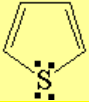
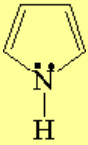
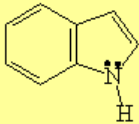
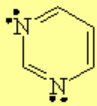
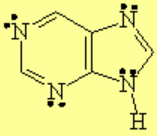
All molecules below possess 4n+2 pi electrons, are conjugated and cyclic systems and you may check that are planar. All of them are examples of aromatic compounds.
 |
 |
 |
 |
|---|
| | | |
| Benzene | Naphthalene | Anthracene | Phenanthrene |
 |
 |
 |
 |
|---|
| | | |
| [18]-Annulene | Pyridine | Furane | Thiophene |
 |
 |
 |
 |
|---|
| | | |
| Pyrrole | Indole | Pyrimidine | Purine |
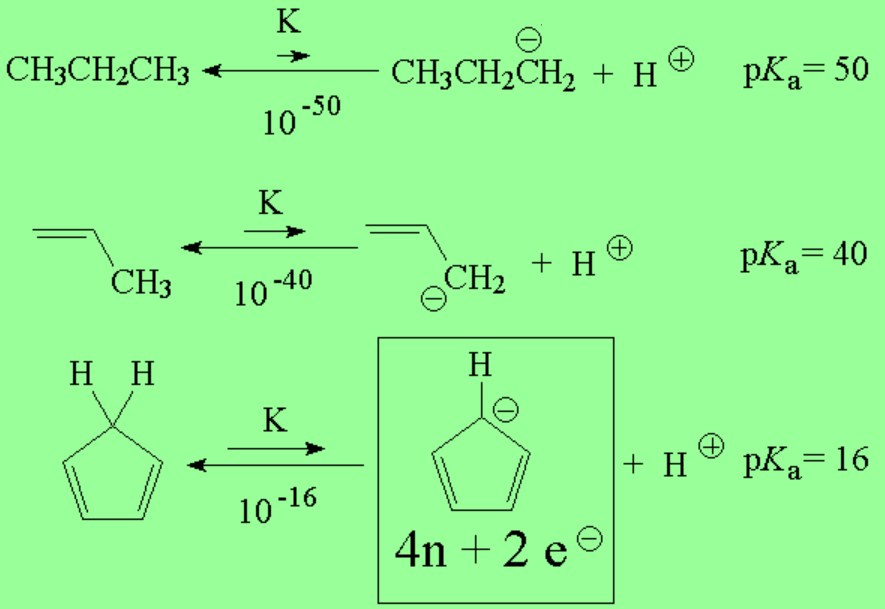
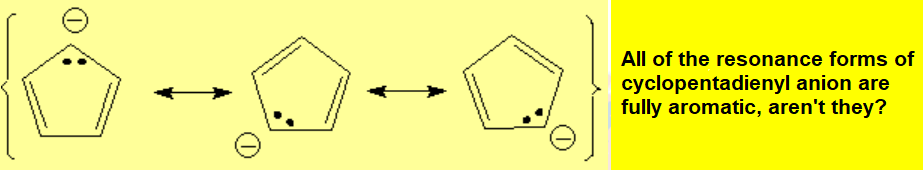
It happens that the sp3 carbon of cyclopentadiene is much more acidic than expected (as acidic as an alcohol!!!). One can explain that as usual, looking at the conjugate base, the cyclopentadienyl anion: Don't you realize that it is aromatic and thus exceptionally stable?
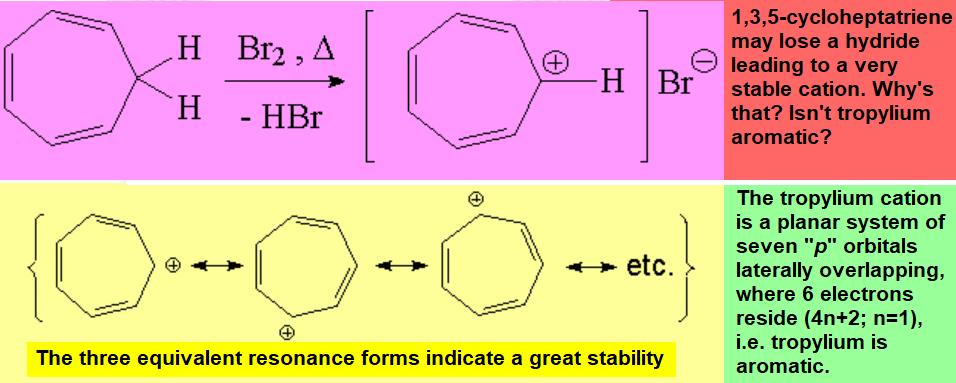
CYCLOHEPTATRIENYL CATION
(TROPYLIUM CATION)