| 1) Electron density:
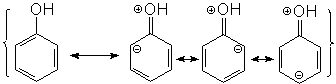
The electron lone pairs of oxygen delocalizes towards the ring and increases electron density at ortho and para positions.

|
The zone with the highest electron density is in fact the oxygen because is the most electronegative atom in the molecule.
Within the ring, the electronic density concentrates in the ortho and para positions. The resonance forms with charge separation won't contribute much to the resonance hybrid.
But they allow us to explain why the negative charge concentrates in ortho and para positions.
The resonance forms with charge separation won't contribute much to the resonance hybrid.
But they allow us to explain why the negative charge concentrates in ortho and para positions. |
2) Relative reaction rate
Phenol reacts 1000 times faster than benzene. The OH group activates the aromatic ring in SEAr and lowers the activation energy. The intermediate bezenonium ion is more stable when the OH is attached to it.
3) Direction of substitution
Nitration takes place almost exclusively on ortho-para positions. This means that the ortho and para benzenonium ions must be much less unstable that the meta one. The resonace forms of each ion give us the answer.
ortho substitution
 |
|
The positive charge delocalizes along the ortho and para positions. In the third resonance form the positive charge lies on the carbon supporting the OH group. The oxygen is thus able to further delocalize the positive charge by sharing its electron lone pairs.
Therefore, the resonance hybrid is described with four resonance forms.
|
|
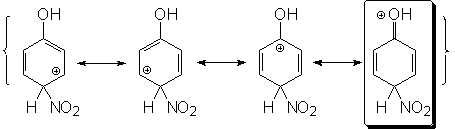
para substitution
|
|
The reasoning is similar to that used for the ortho position.
|
|
meta substitution

|
|
The positive charge in no case lies on the carbon attached to the OH group that is unable to further delocalize it. Therefore, the resonance hybrid is described with only three resonance forms and is more unstable than the preceding cases.
|
|
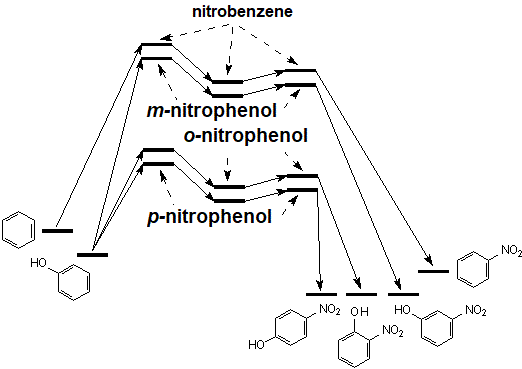
The energy diagram of phenol nitration is qualitatively similar to benzene's.
Phenol reacts faster than benzene. That experimental fact suggests that benzene reaction pathway has to be the highest. From the three possible pathways of phenol, ortho, para and meta, the latter should be the second highest because is the least stabilized by resonance (see above).
The major isomer is para and hence, its pathway has to be the lowest in energy. Experimentally, the ratio ortho-para is 40:58 but there are two equivalent ortho positions. We have to take out this statistical factor in order to compare true rate ratios.
Therefore, the para position reacts 58/20 times as fast as the ortho, i.e. 2.9:1
|
|