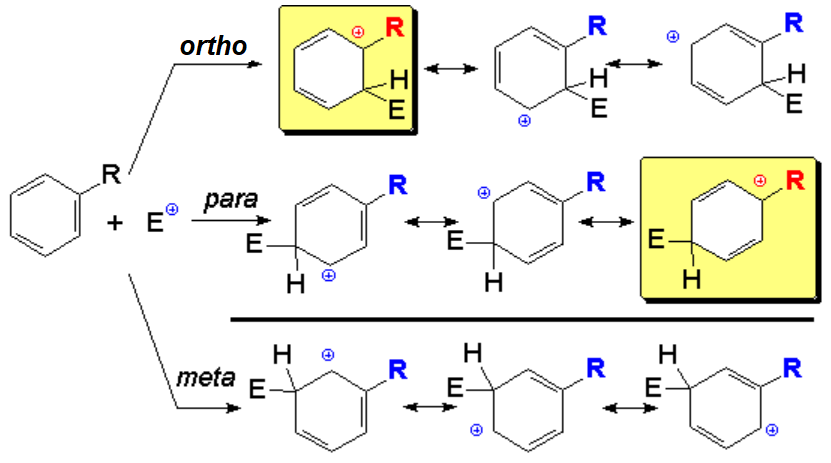
In a second SEAr, the incoming functional group can attach to the ring at ortho, meta or para position relative to the pre-existing R group.
In the intermediate benzenonium ion the pre-existing R group will or will not interact directly with the developed positive charge, depending the position where the new substituent attaches to.
The experimental regiochemistry of the reaction can be easily explained in terms of favorable or unfavorable interactions of the R group with the positive charge.
Regardless of the activating or deactivating nature of the R group, if it is able to stabilize the positive charge (alkyl, halogen, OR, NR2), the second SEAr will be directed to ortho-para positions.
Should the R group be unable to stabilize the positive charge (CF3, NO2, CO2R, +NR3), the second SEAr will be effected at meta position.