SYNTHETIC STRATEGIES IN SEAr
Polysubstitution on benzene or other aromatic systems has to be carefully planned.
One has to be fully aware of the activation/deactivation and orientation properties of the functional groups to be attached.
Unlike an arithmetic operation, in a synthetic sequence, the order in which the synthesis steps are planned DOES ALTER the product.
The functional groups can be properly transformed one another and consequently their activation/deactivation and orientation properties can be adequately changed.
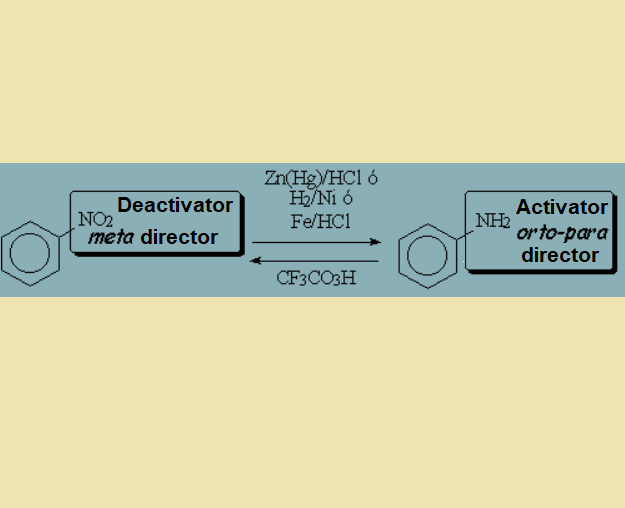
Conversion of a NO2 group, strong deactivator and meta director, into a NH2 group, activator and orto-para director.
And viceversa...
But beware of the acidic media that transforms NH2 into NH3+!!!
All your plans will go to the sink...
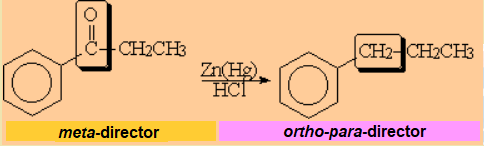
Conversion of a ketone group, deactivator and meta director into an alkyl group, activator and orto-para director.
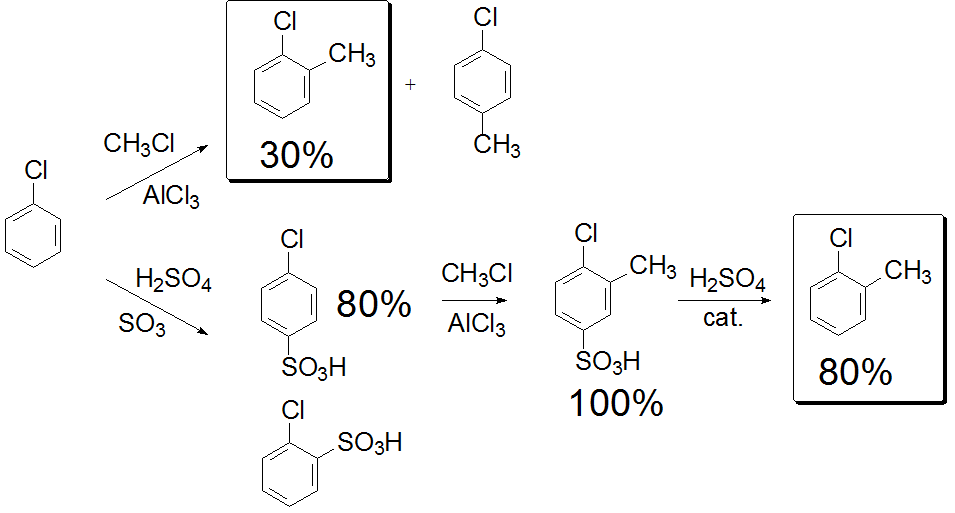
Sulfonation allows the momentary blocking of the para position.
One can get ortho-substituted products in a more efficient way by protecting or momentarily blocking
the para position by sulfonation.
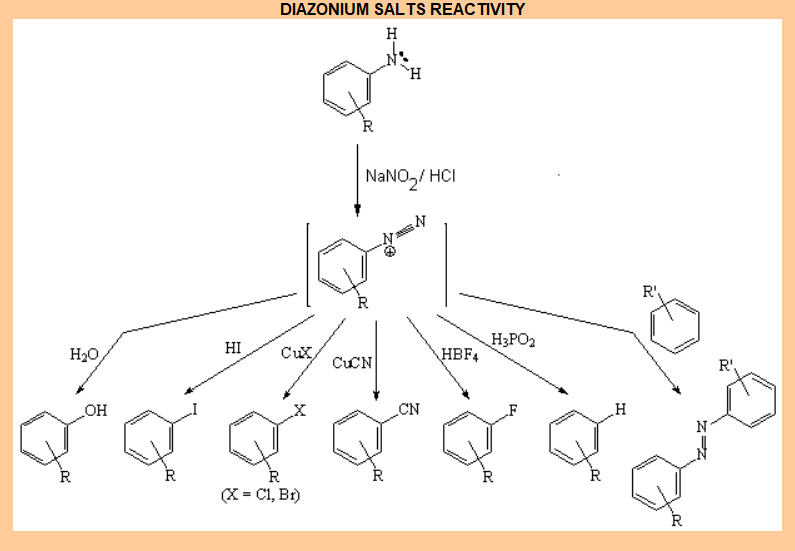
Transformation of a NH2 group into a diazonium salt.
Diazonium salts can be replaced by umpteen other groups.