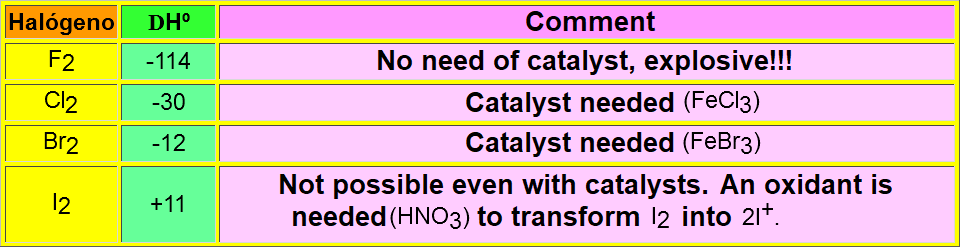
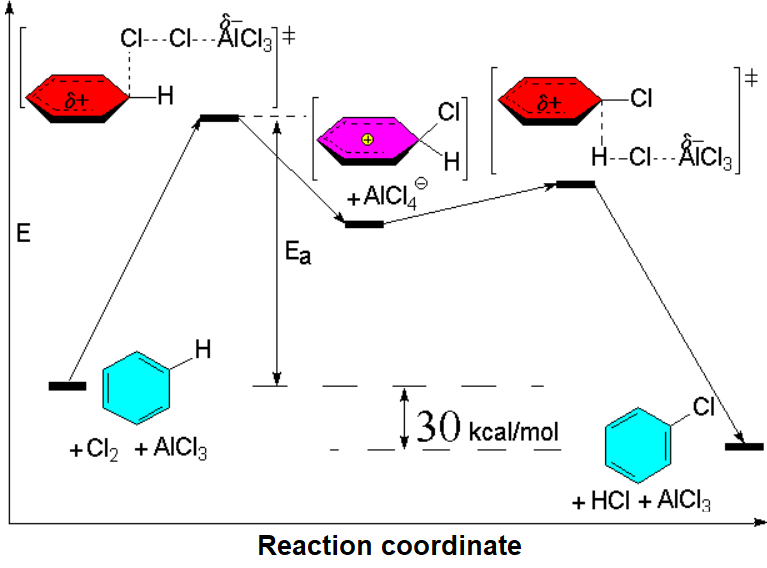
deltaHº= (deltaHº)Cl-Cl + (deltaHº)H-C6H5 -[(deltaHº)H-Cl + (deltaHº)ClC6H5] = = 58 + 111 - (103 +96) = -30 kcal/mol
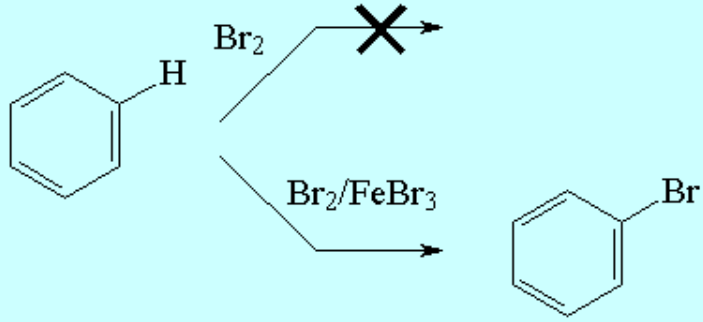
The SEAr reactions are usually thermodynamically favorable.
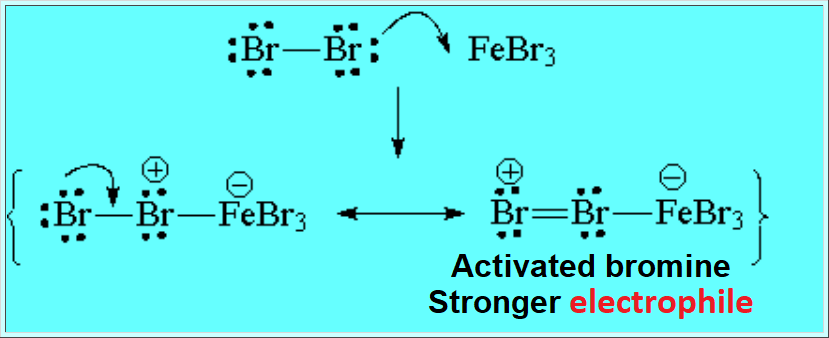
The use of catalysts is mandatory in order to surmount the activation energy and get a reasonable reeaction rate.