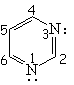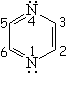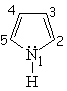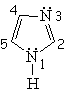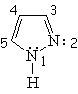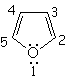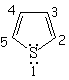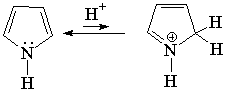The heteroaromatic compounds may contain various heteroatoms within their rings.
The limits are as always in your imagination...
Hückel put forward rules for aromaticity and antiaromaticity:
A system of pi bonds is aromatic when:
1) conjugated
2) cyclic
3) planar
4) holding 4n + 2 electrons.
IMPORTANT: If it holds 4n electrons instead, the pi system is antiaromatic
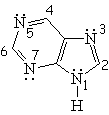
All the following molecules comply with the previous conditions of aromaticity.
In order to account for the 4n+2 pi electrons, the heteroatoms put, or don't, their electron lone-pairs into play.
 |
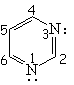 |
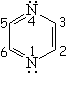 |
 |
|---|
| | | |
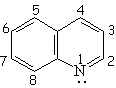
| Pyridine | Pyrimidine | Pyrazine | Quinoline |
 |
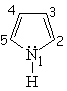 |
 |
 |
|---|
| | | |
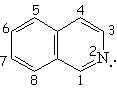
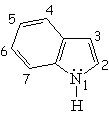
| Isoquinoline | Pyrrole | Indole | Purin |
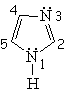 |
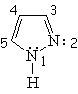 |
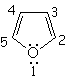 |
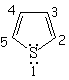 |
|---|
| | | |
| Imidazole | Pyrazole | Furane | Thiophene |
The electron lone-pairs of the heteroatoms may or may not be shared in the 4n+2 electron pool, one of the conditions for aromaticity.
All depends on the struture.
What can one deduce from the following basicity data?
pKb = 8.8
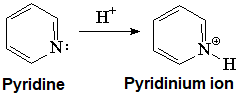
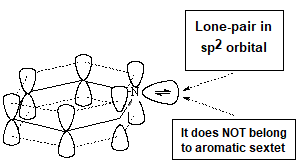
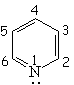
Pyridine is definitely basic,
isn't it?.
This fact indicates that the electron lone-pair of nitrogen MUST NOT BE CONTRIBUTING to the aromaticity. The six electrons contained in the six "p" orbitals comply by themselves with the 4n+2 (n=1) electron Hückle's rule.
pKb > 18
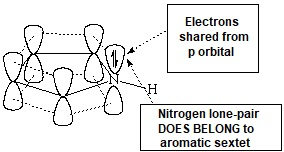
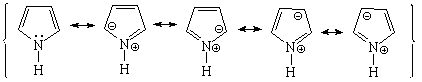
Pyrrole is not basic at all.
This is a clear indication that the electron lone-pair of nitrogen DOES CONTRIBUTE to the aromaticity, is heavily delocalized and cannot thus react with an acid. Hückle's 4n+2 (n=1) rule is complied by the four electrons provided by the four carbons and the two electrons shared by the nitrogen.
The eletronic structure of both rings allows us to understand their different basicity properties:
Concerning reactivity, one may wonder whether they are more or less reactive towards SEAr than benzene. What is your best educated guess?
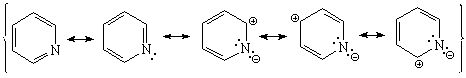
Pyridine's nitrogen withdraws electron density from its ring.
Pyridine is expected to be LESS reactive than benzene regarding SEAr.
Pyrrole's nitrogen is contributing with its two electrons to aromaticity. Its role is opposed to that showed in pyridine.
Pyrrole is expected to be MORE reactive than benzene regarding the SEAr.