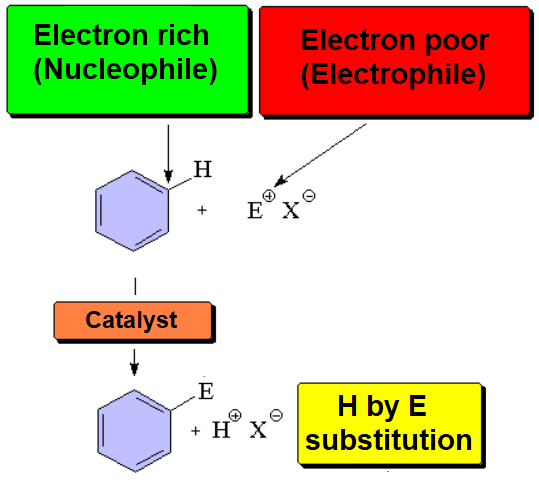
An electron-deffective reactant attacks (is attacked by) benzene's pi cloud, electron rich, rendering a new benzene derivative (aromaticity is preserved) where a hydrogen ends up substituted by a functional group.
Such kind of reaction is called Aromatic Electrophilic Substitution (SEAr).
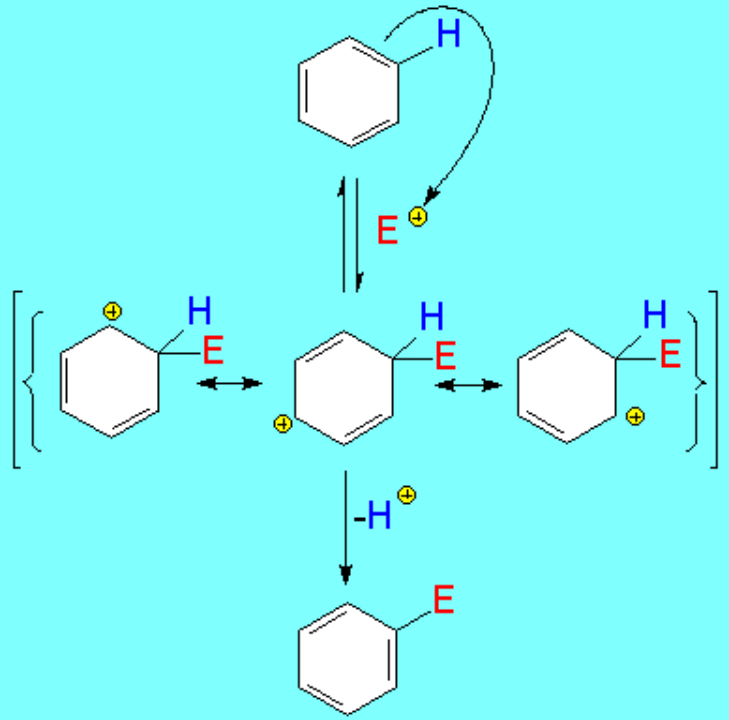
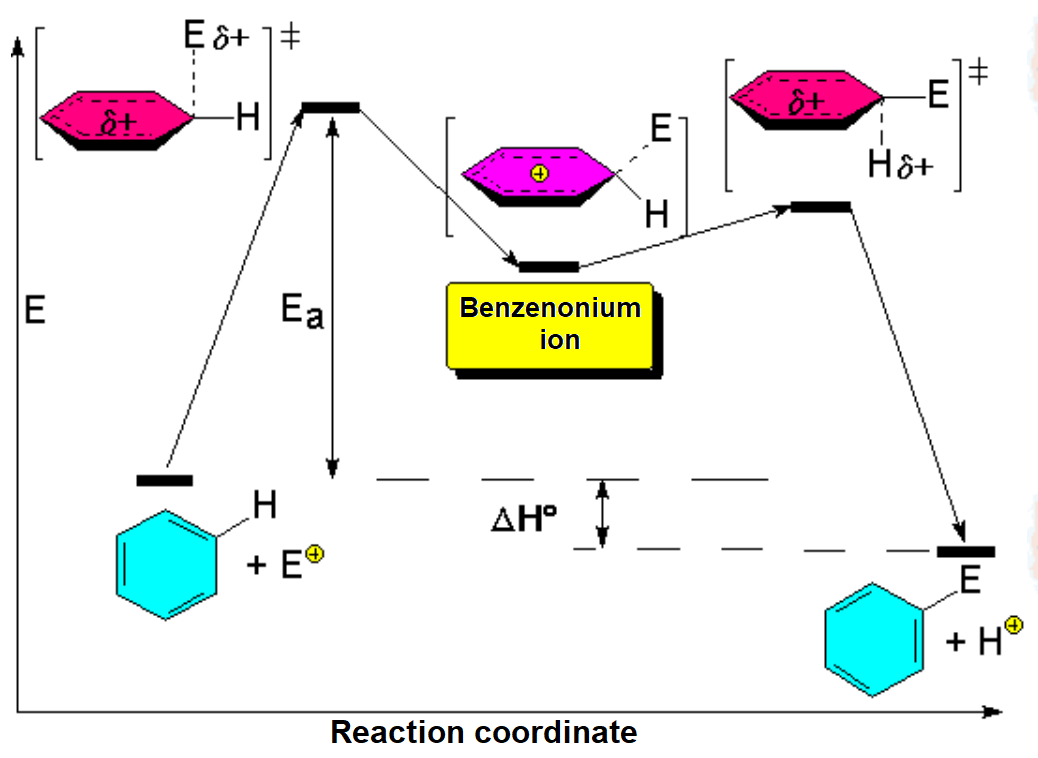
The reaction takes place when the benzene's HOMO (Highest Occupied Molecular Orbital) turns over electrons to the electrophile's LUMO (Lowest Unoccupied Molecular Orbital) leading to a positively-charged non-aromatic intermediate (benzenonium ion).
Bencene's reacting carbon swaps its initial trigonal sp2 hybridization to the tetrahedral sp3 in the benzenonium ion intermediate.
The intermediate benzenonium ion, although higher in energy due to its aromaticity loss, bears a certain stability because the positive charge can be somewhat delocalized over the ortho and para positions relative to the attack site.
The loss of a proton regenerates the aromaticity.
The lost proton is captured by E+'s counterion.
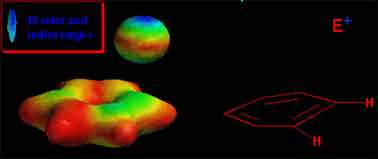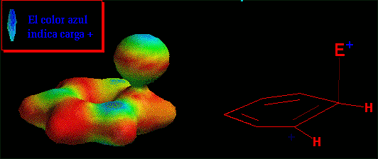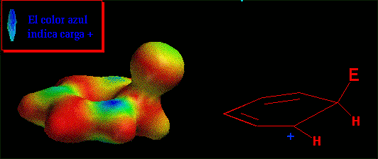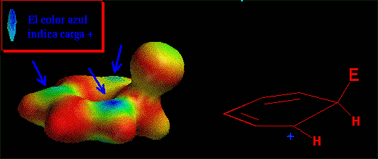
Have a look at the attack in movement. The electrophile approaches the aromatic ring in a perpendicular trajectory to the ring plane, just in the very direction of the "p" orbital of the reacting carbon.
As long as the new bond is being formed, the reacting carbon gradually changes from a trigonal stance to a tetrahedral layout.
The energy diagram of the SEAr is as follows:
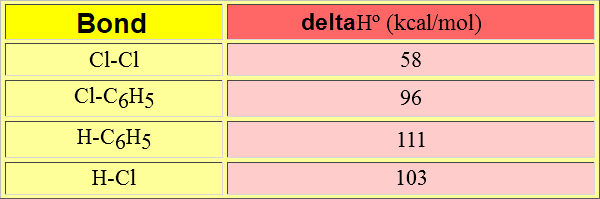
Which is the overall energy balance for the SEAr with chlorine for instance?:
deltaHº = (deltaHº)Cl-Cl + (deltaHº)H-C6H5 -[(deltaHº)H-Cl + (deltaHº)ClC6H5] = = 58 + 111 - (103 +96) = -30 kcal/mol
The SEAr reactions are usually theromodynamically favorable.
Catalysts are generally used in order to lower the activation energy and get the reaction proceeding at a reasonable rate.