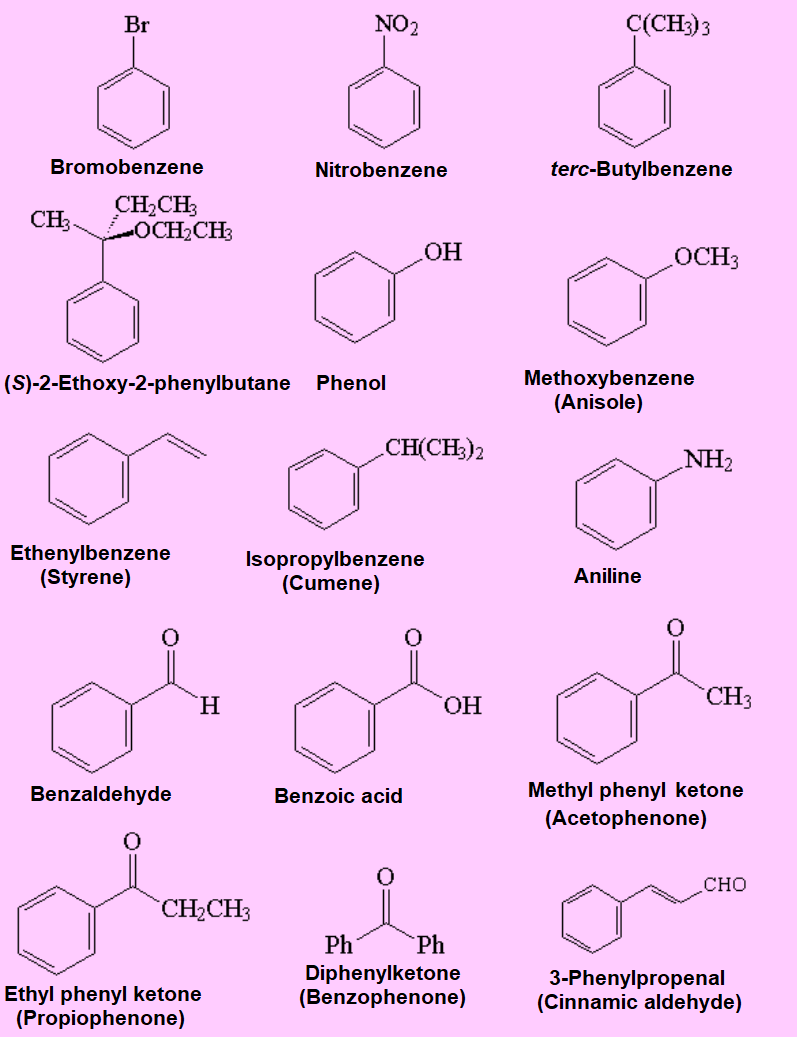
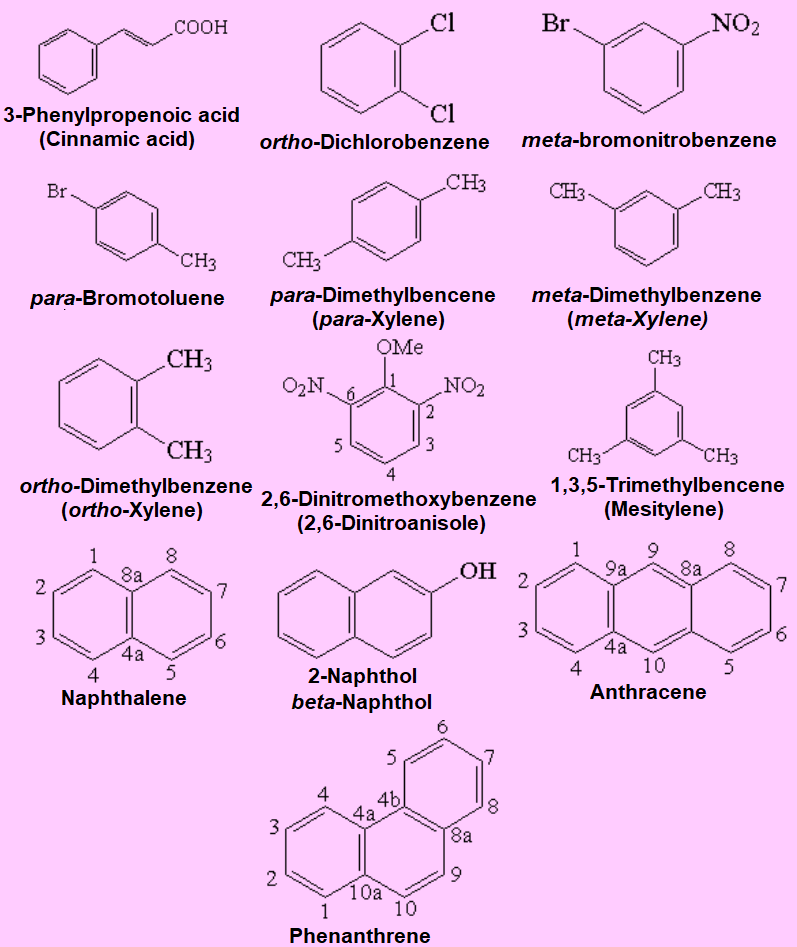
Nomenclature rules are quite complex. We only dare relatively simple cases and names. Look at the following representative examples.
Try to remember the trivial names that certain functional groups get when bonded to a benzene or other aromatic systems.
Observe how the rings are numbered and the special designation of positions 2, 3 and 4 on a benzene as ortho, meta and para, respectively.