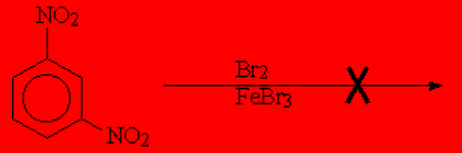The attachment of three groups to a benzene by consecutive SEAr reactions is not easy a task at all.
One must start by the least deactivating groups because, if not, the last reaction would become impossible.
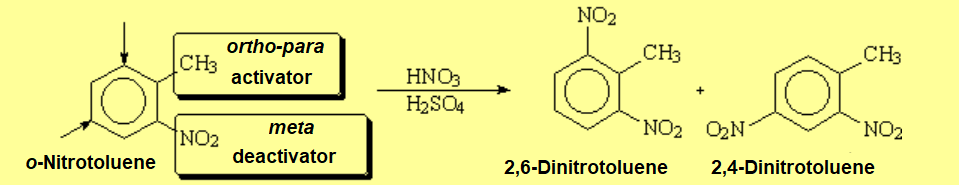
How do you explain this result?
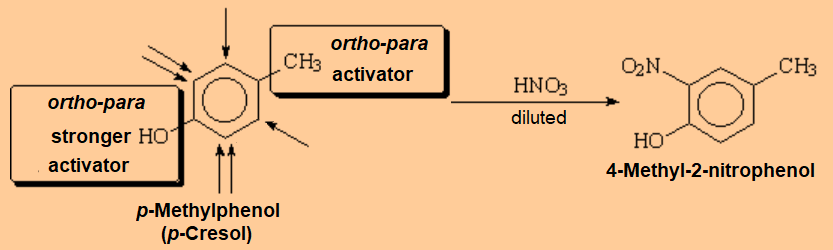
Piece of cake! The two groups direct to the very same position.
If two substituents conflict with their directing trends, the activator or the weaker deactivator beats the other.
How come this nagative result?
If the benzene has two deactivators in the first place, the third SEAr can be even impossible to perform.
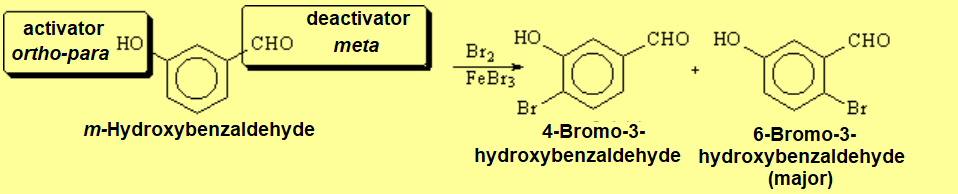
Last but not least, how can one explain this result?
If two groups are already in meta position to each other, the third substitution won't go to the position in between them due to heavy steric congestion.