ACID - BASE PROPERTIES OF ALCOHOLS
The O-H bond is stronger (deltaHº = 104 kcal/mol) than the C-H (deltaHº = 98 kcal/mol) but, in turn, it is much easier to heterolyticaly break.
How come?
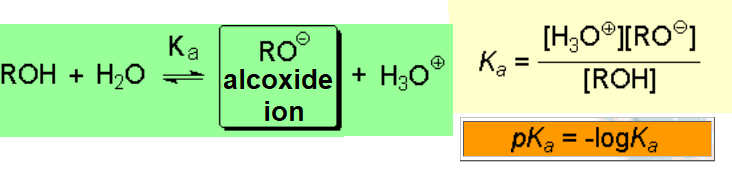
The heterolytic cleavage of an O-H bond produces an alkoxide where the negative charge is placed on an oxygen, much more electronegative than a carbon.
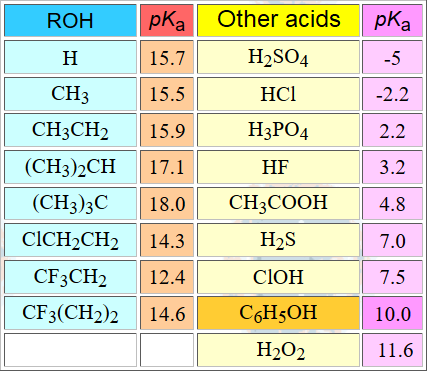
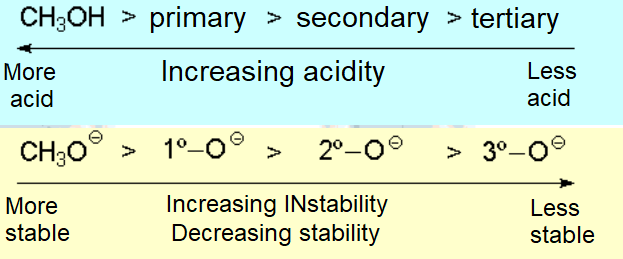
The acidity of an alcohol can be qualitatively understood in terms of the relative stability of the resulting alkoxide.
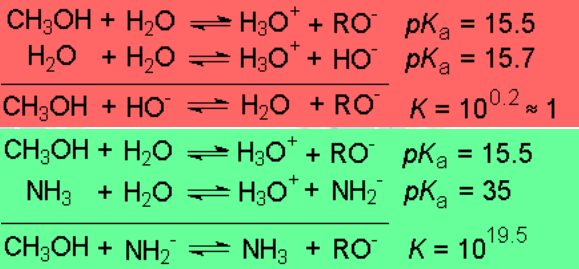
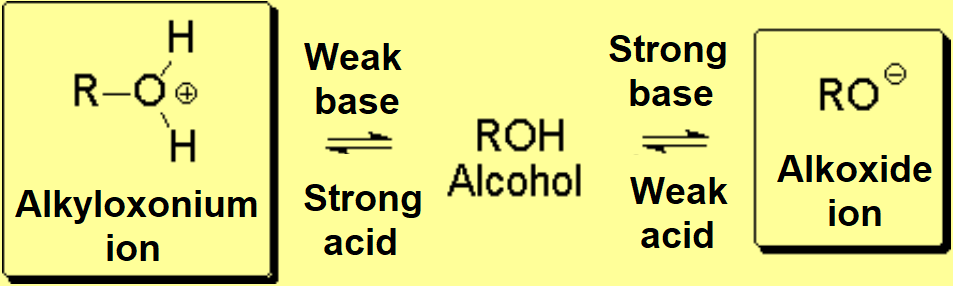
One needs relatively strong bases to quantitatively convert alcohols into their conjugated bases, the alkoxides:
Alcohols are amphoteric because the lone electron pairs on the oxygen can be shared with an external acid, provided it is a strong one.