PREPARATCION OF ALCOHOLS BY ORGANOMETALLIC REAGENTS
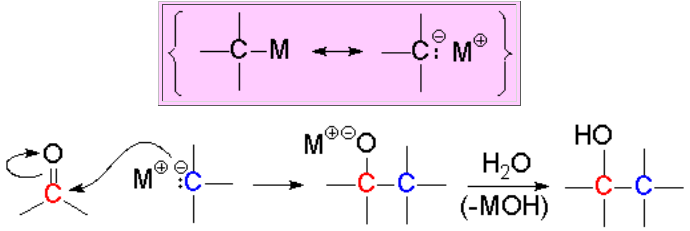
A carbon atom bonded to a metal, much less electronegative, is turned into an electron rich center and an excellent nucleophile.
The C-Metal bond is heavily polar.
The most common reagents are the organolithium and the organomagnesium (Grignard) derivatives.
Víctor Grignard (1871-1935): French Chemist. In 1910 discovered the organomagnesium reagents that were called after him. He won the Noble Prize in 1912, shared with Sabatier. He authored a chemistry treatise of 20 volumes.
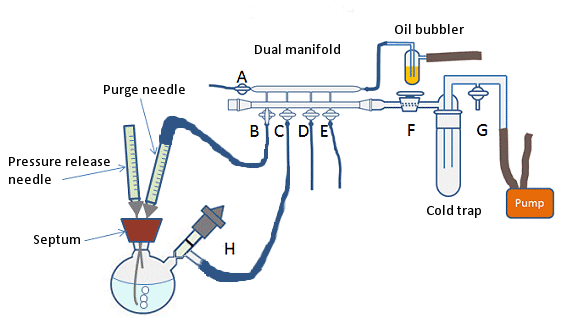
Organometallics have to be used right after their preparation bacause they are so reactive that get easily and violently destroyed by oxygen and moisture. Their preparation and use must be carried out under inert atmosphere (N2 or Ar). Image
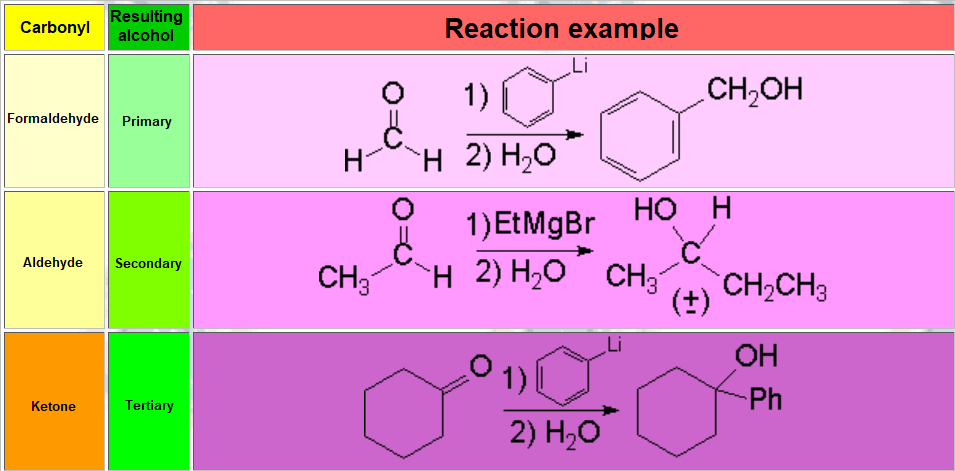
Depending on the structure of the carbonyl group, the resulting alcohol will be differently substituted: