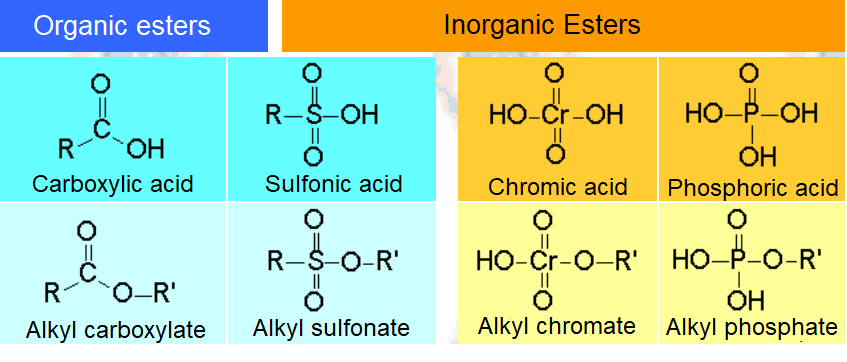
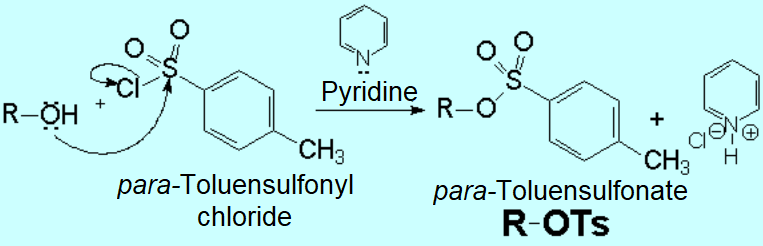
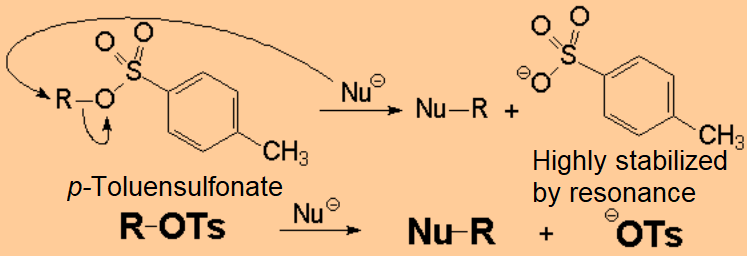
One of the most useful reaction of alcohols is its conversion to an ESTER, either organic or inorganic one.
The reaction is performed from the alcohol and the corresponding organic or inorganic acid.
ACID + ALCOHOL = ESTER + WATER
The central atom of the different acids is surrounded by so many oxygen atoms that is heavily electron defficient.
It can thus be attacked by the electron lone pairs of the alcohol OH.
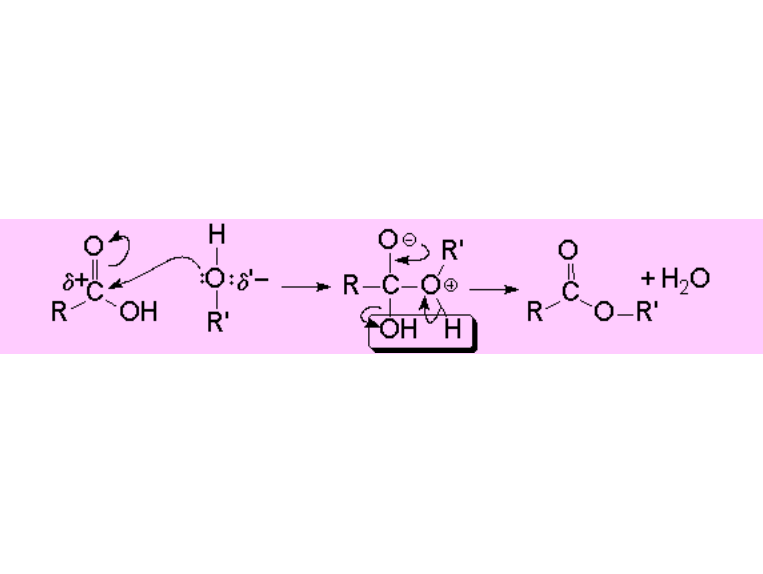
We'll see into the complete mechanism elsewhere but, for the time being, we can schematically look at the ESTER formation: