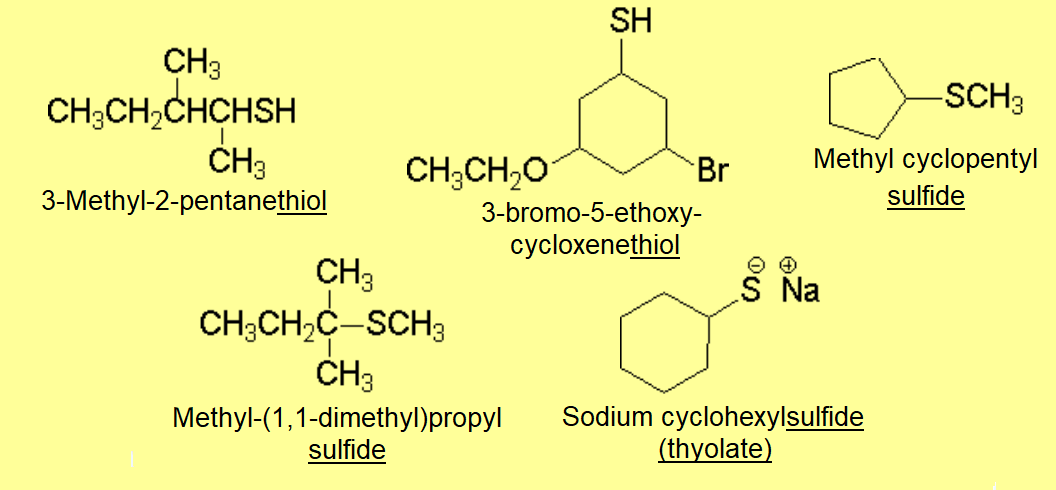
 However, dimethylsulfide displays a higher b.p. than its oxygen homolog.
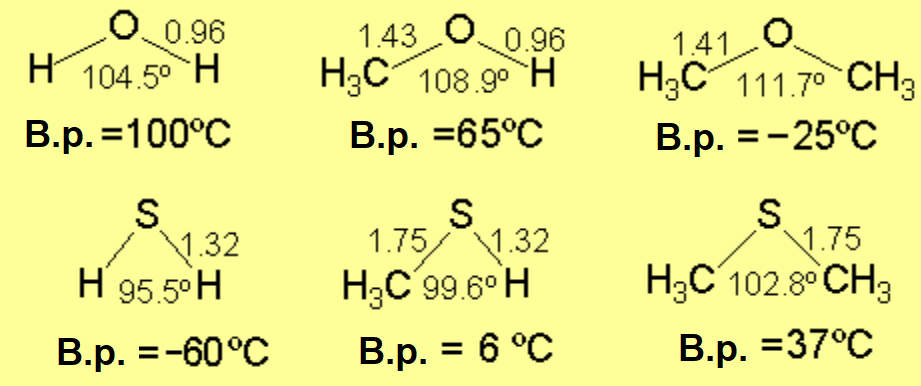
However, dimethylsulfide displays a higher b.p. than its oxygen homolog.
This should be a consequence of the larger size and polarizability than oxygen.
No hydrogen bonding is possible.
The S-H···S hydrogen bond is weaker than the O-H···O.
The lower b.p. of hydrogen sulfide and methylthiol as compared to water and methanol, respectively, is proof of that.
 However, dimethylsulfide displays a higher b.p. than its oxygen homolog.
However, dimethylsulfide displays a higher b.p. than its oxygen homolog.