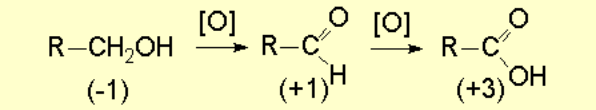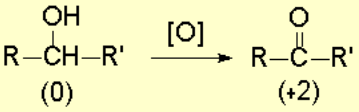One may obtain alcohols by oxidation of other less oxidized functions.
It is easy to imagine the other-way-around process:
In a primary alcohol, the carbon bearing the OH group has a formal oxidation state of -1 what allows it to undergo multiple oxidation possibilities.
Many oxidation reagents are inorganic salts, like KMnO4 or K2Cr2O7, only water soluble.
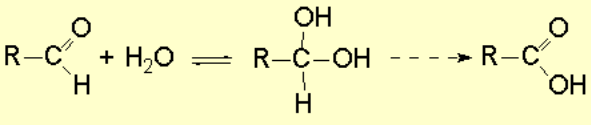
Water forms hydrates with aldehydes thus making the second oxidation step be easier than the first.
It is thus very difficult to stop the alcohol oxidation at the aldehyde stage.
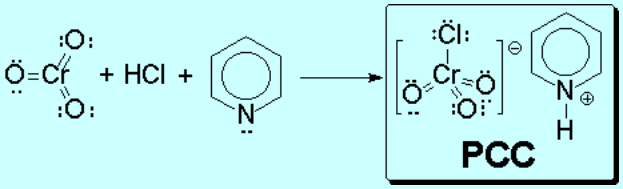
In order to accomplish it, one has to use reagents with at least an organic part, making them soluble in organic solvents and hence avoiding the presence of water.
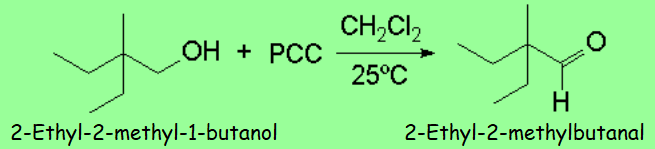
Pyridinium Chlorocromate
PCC
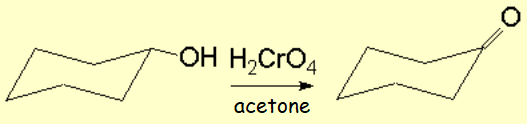
PCC is soluble in organic solvents.
Its reaction with primary alcohols is selective and does stop at the aldehyde.
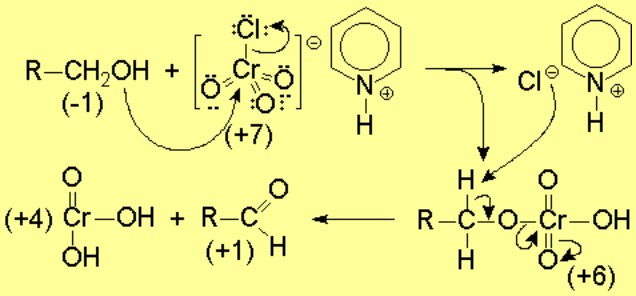
A plausible mechanism for this reaction is the following:
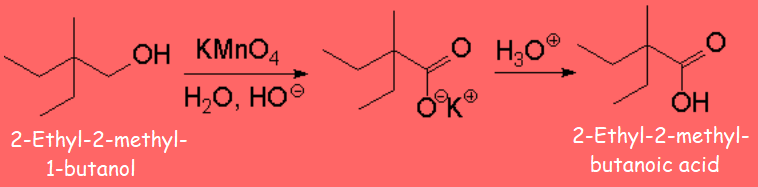
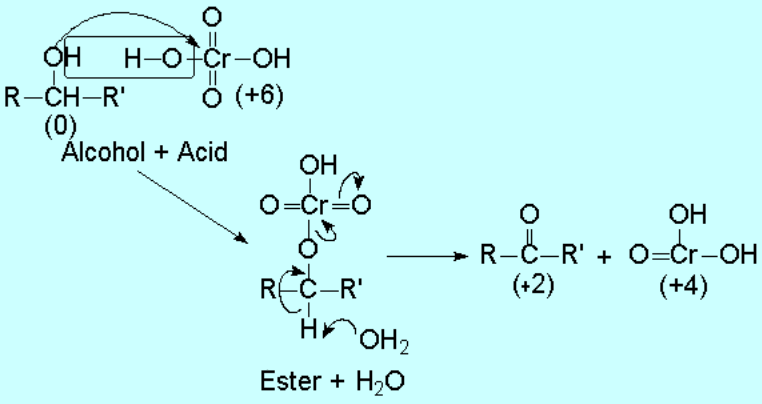
They can be transformed into ketones, without breaking the molecule, something that can easily happen if reaction conditions are harsh.
A probable mechanism for this reaction can be as follows:
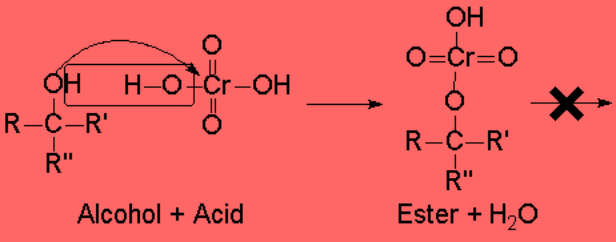
Tertary alcohols cannot be oxidized without breaking the molecule because they lack one hydrogen which could be eliminated.
In fact, they are pretty resistant to oxidation.