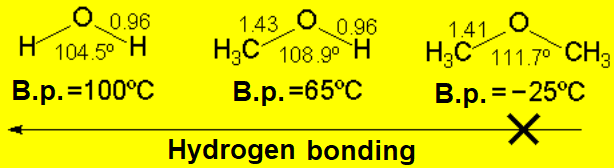
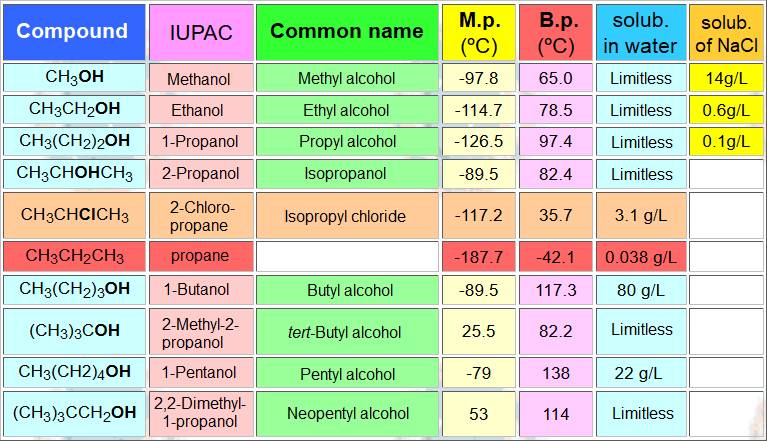
The hydroxyl group confers polarity to the molecule and the possibility of forming hydrogen bonds.
The carbon part is non-polar and hydrophobic.
The larger the carbon chain, the lower the alcohol solubility in water and the more soluble in non-polar solvents.