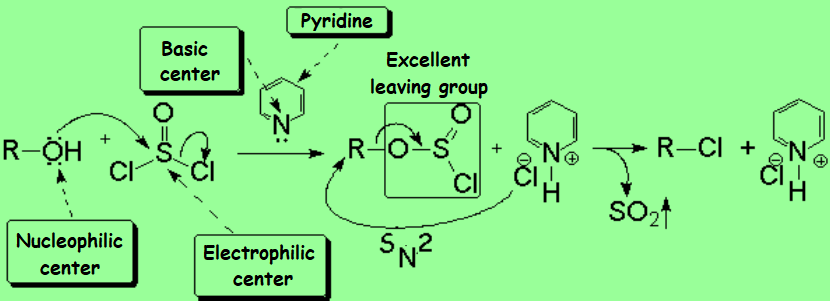
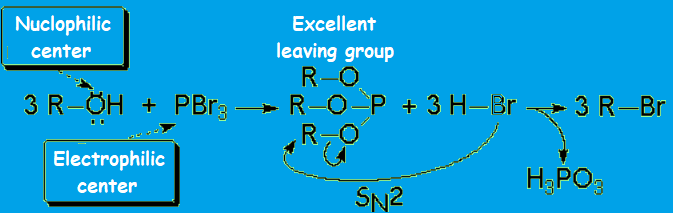
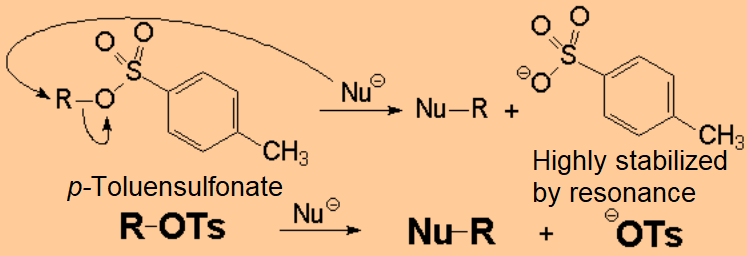
For instance, the alcohol can be treated with a strong mineral acid to induce the protonation of the OH.
Once the OH is protonated, the leaving group turns into a water molecule:
Protonation of the OH involves a severe increase in the C-O bond polarization, making much easier the spontaneous heterolitic cleavage, leading to a carbocation.
The production of a carbocation always bring rearrangement problems, i.e. the formation of UNEXPECTED products.
Look carefully!!!
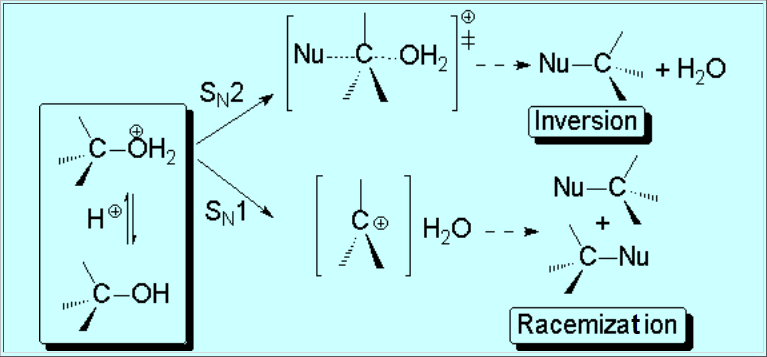
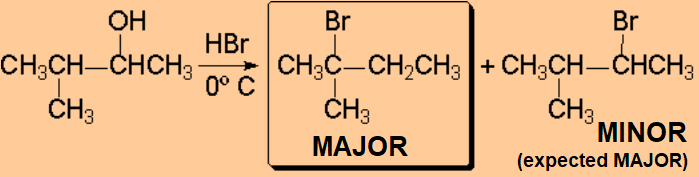
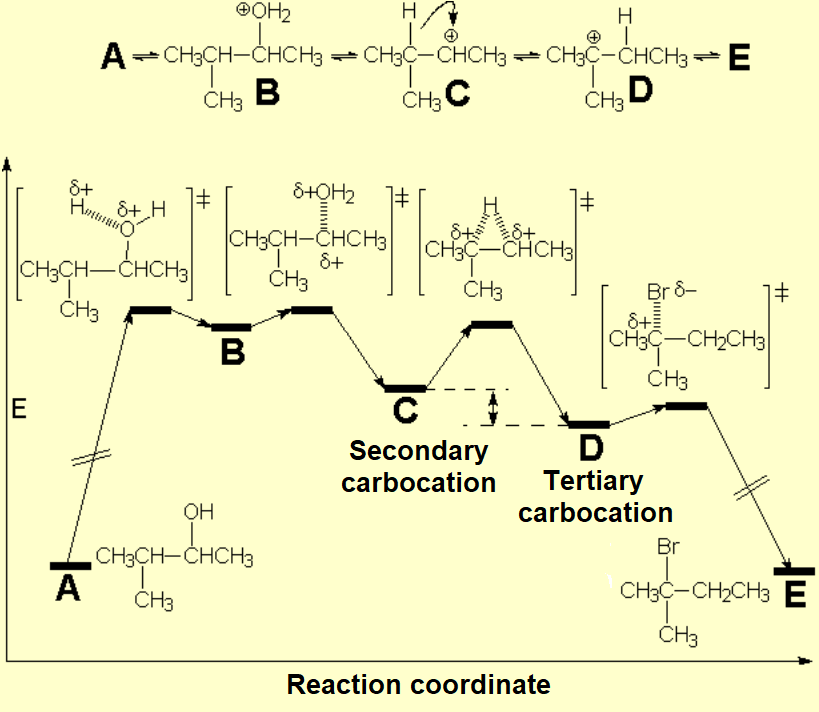
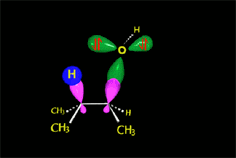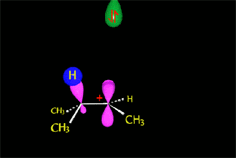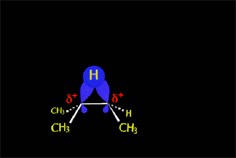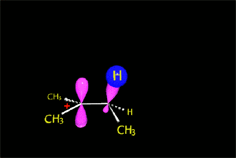 How come the rearrangement?
How come the rearrangement?