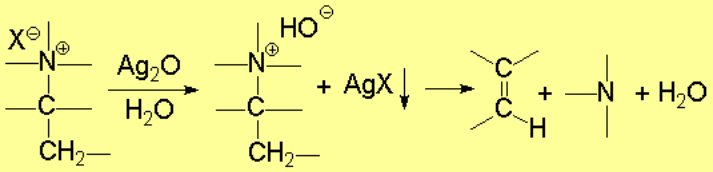Degradation is one of the most important reactions of amines.
It leads to olefins.
The general scheme is the following:
August Wilhelm von Hofmann (8 April 1818 – 5 May 1892) was a German chemist.
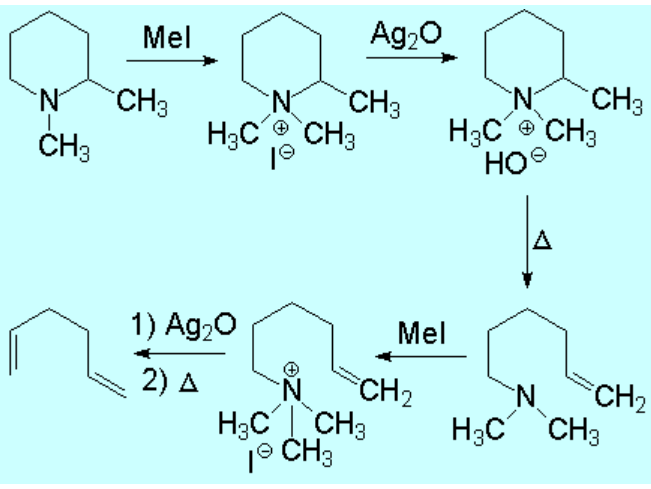
Look at the following example where a 'double' degradation takes place:
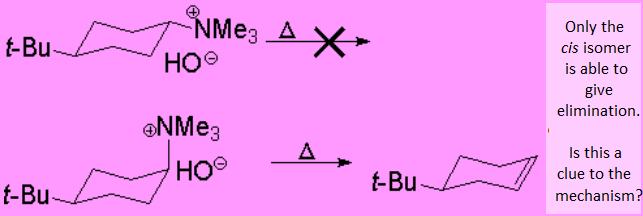
What is Hofmann degradation's mechanism?
Let's see whether the following experimental result gives you a clue!!!
The large tert-butyl group blocks the conformational equilibria to its equatorial arrangement.
The preferred conformer of the cis isomer does not have any hydrogens in anti arrangement relative to the NMe3 group. That's why it doesn't react, because the mechanism is E2.
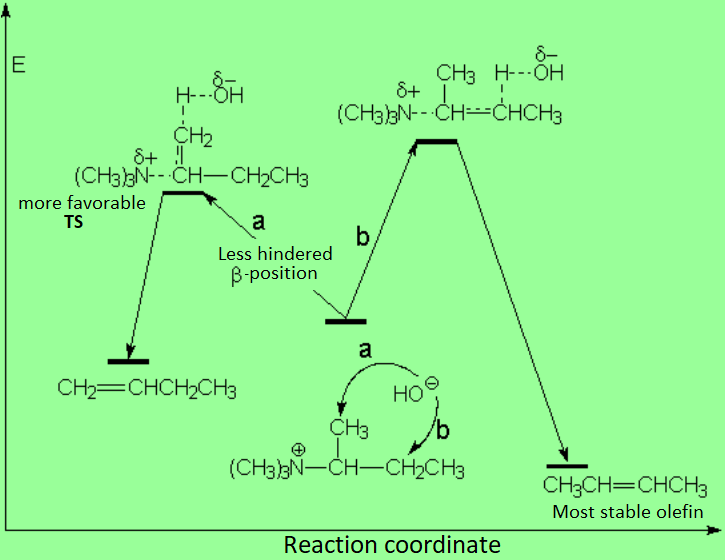
The reaction is kinetically controlled leading to the monosubstituted olefin which is less stable.
The more substituted the olefin, the more stable.