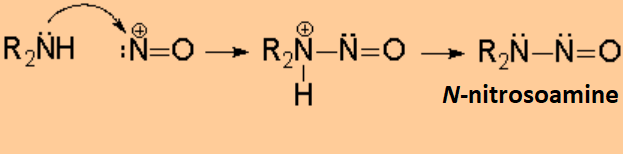
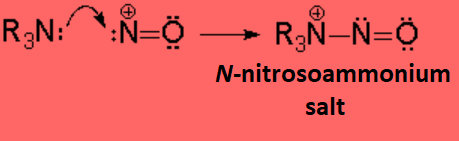
A very important reaction of amines takes place with nitrous acid.
Such process is called nitrosation.
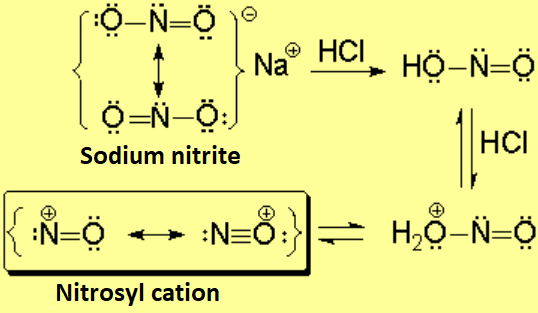
Nitrous acid decomposes in acidic medium, releasing the nitrosyl cation, a very strong electrophile.
The nitrosyl cation is attacked by the amine leading to a N-nitrosyl ion, that may evolve in a different fashion depending whether the amine is primary, secondary or tertiary.
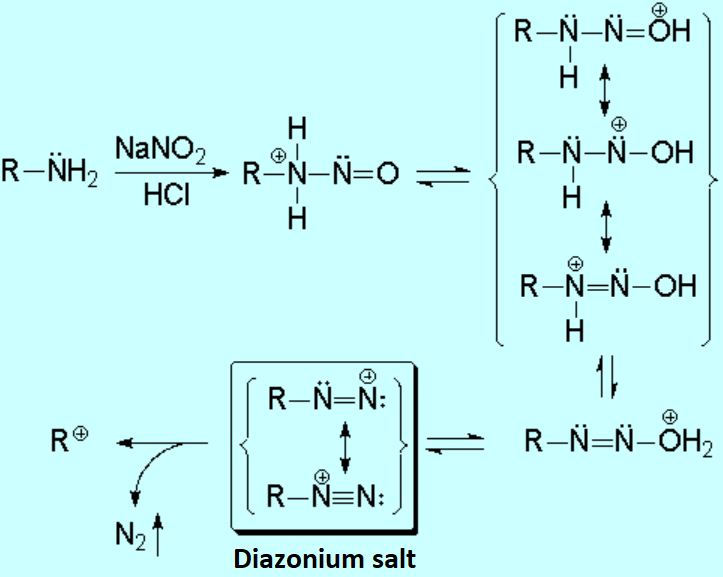
The two hydrogens on the nitrogen of the primary amine allows for the elimination of a water molecule.
A very reactive diazonium salt is thus formed, which tends to easily loose a nitrogen molecule, yielding a carbocation that will react with any present nucleophile.
The aliphatic diazonium salts are extremely unstable and quickly decompose. The so-formed carbocation uncontrolably reacts with any present nucleophile (for instance water, chloride, etc.) leading to a mixture of alcohols, alkyl halides and even alkenes by elimination.
AROMATIC PRIMARY AMINES (ANILINES)
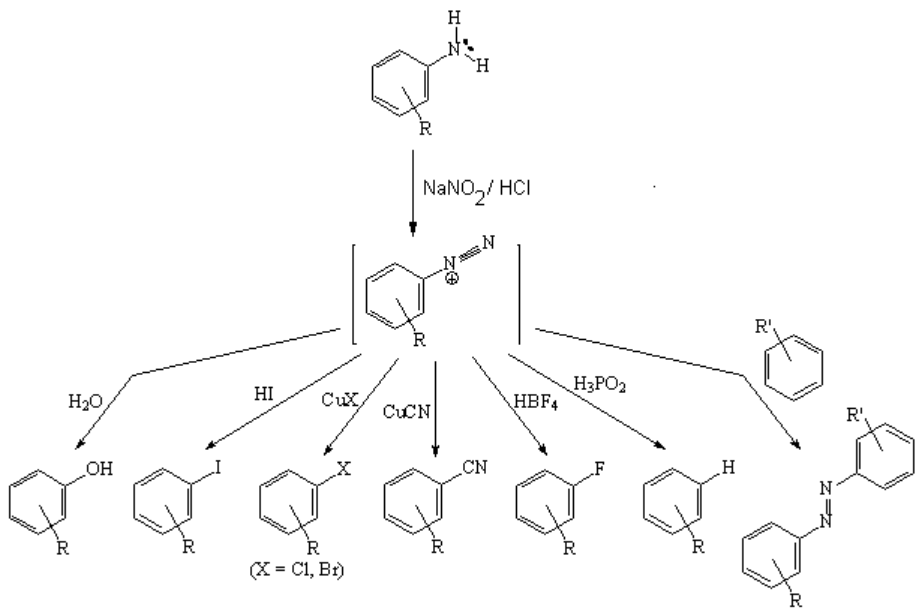
The most important reaction of non-substituted anilines is their transformation into diazonium salts, relatively stable compounds intermediate on the way to numerous aromatic derivatives.
The N-nitrosoamines are very dangerous because they are proved potent carcinogenic agents.
Nitrites (E-249 y E-250) are preserving agents for meat and its common derivatives like sausages, etc., that avoid the proliferation of microorganisms producing botulinic toxins, lethal for human beings.
The N-nitrosoamines might be produced by an overcooking of nitrite-containing food.
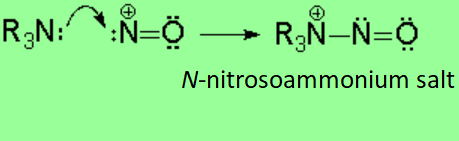
Tertiary amines do not bear any hydrogen on their nitrogen and the N-nitrosoammonium salt cannot evolve. However they easily decompose.
The reaction does not have any synthetic usefulness.