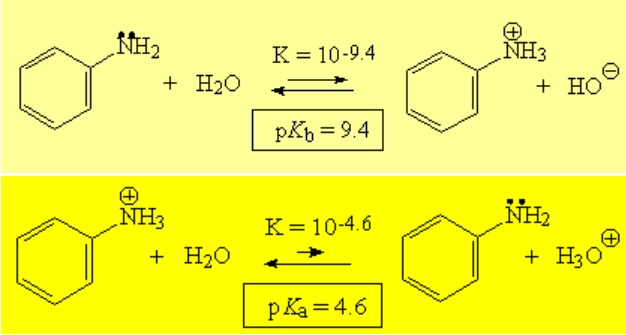
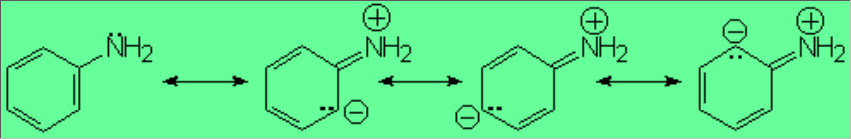
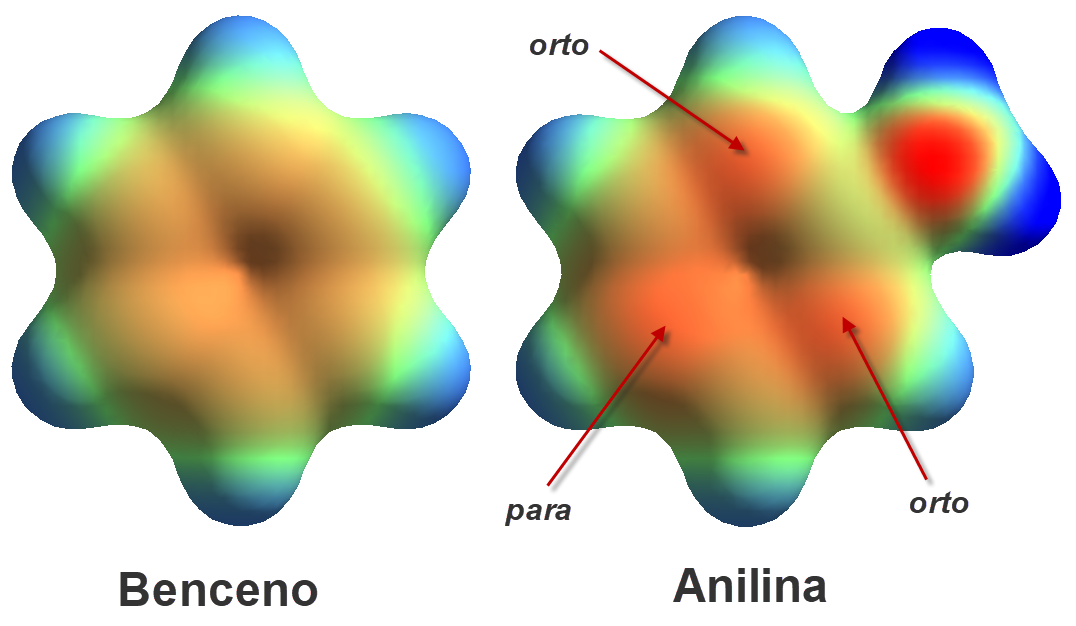
The resonance forms give the explanation because they show us that the electron lone-pair on the nitrogen is delocalized through the ring and thus less available to an external acid. Electron density calculations displays redder (higher density) carbons in ortho and para positions relative to the nitrogen as compared to benzene.