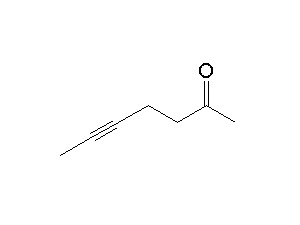
How would you plan the synthesis of the alkyne on the right?
HINT: Use the concept of C=O group's protection/deprotection.
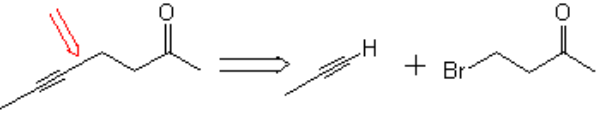
Retrosynthetically speaking, the strategic bond to be created is that marked with the red arrow, taking advantage of the relative acidity of propyne that, once deprotonated, could be alkylated with the appropriate alkyl halide:
But this plan has a serious drawback.
Can you tell what the big problem is?
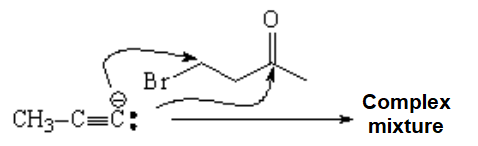
The carbonyl is a strong electrophilic center that competes with the halogenated carbon.
The reaction will thus give an inconvenient mixture of products and be very inefficient.
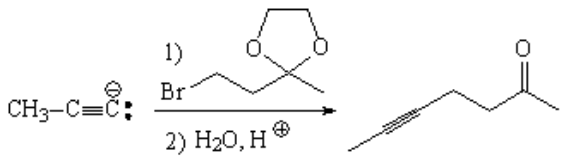
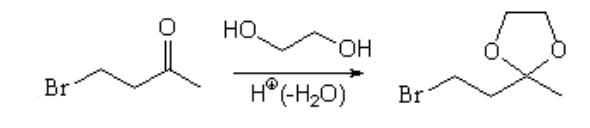
The solution is to protect the carbonyl turning it into a ketal, before performing the nucleophilic substitution of the halogen with the alkynylyde.
At the very end, the carbonyl is recovered by acid hydrolysis.
But, how is the ketal made?
It's very common to use ethylenglycol as a protecting group for aldehydes and ketones.
Ethylenglycol carries in itself the "two alcohol functions" necessary to form a very stable cyclic ketal.