ACID-BASE PROPERTIES OF CARBONYL COMPOUNDS:
ENOLIZATION
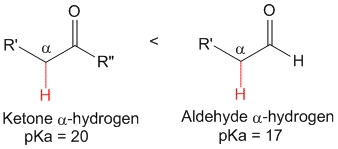
The alpha position relative to a double C=O bond is much more acidic than expected.
As compared to an alkane, the C=O group increases the acidity of its vecinal CH in more than 30 orders of magnitude !!!
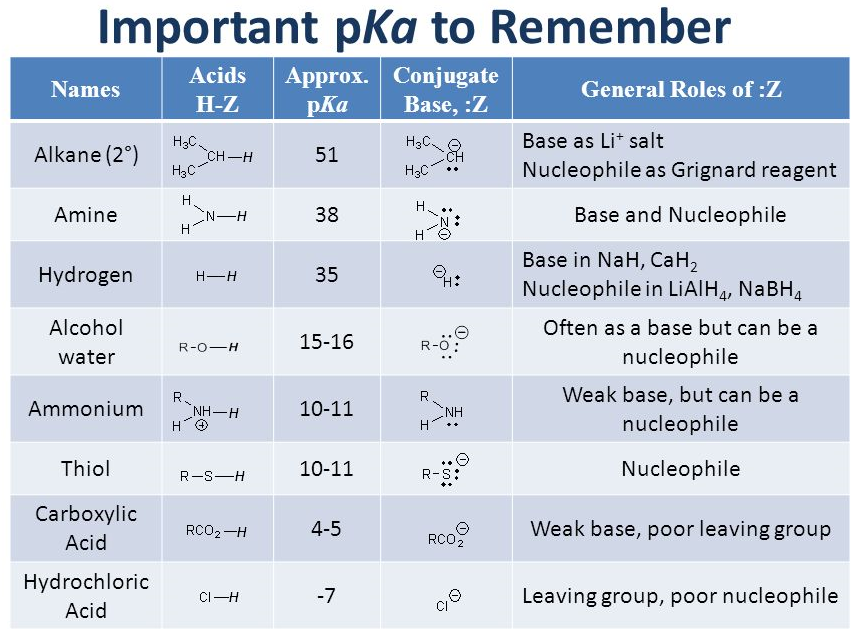
It'd be good if you remember some significant pKa values.
The bases able to deprotonate an alcohol would do it too to the alpha position of an aldehyde or ketone.
But, haven't you realized that aldehydes and ketones keep a base within themselves?
Isn't the oxygen of the C=O group basic enough?
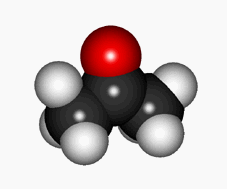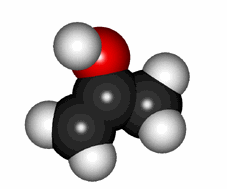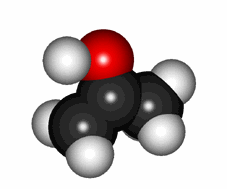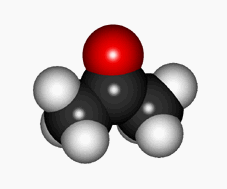
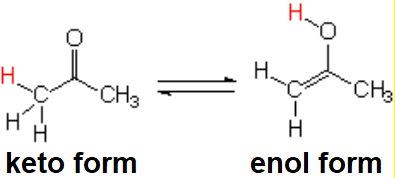
Yes, C=O group's oxygen can pull the hydrogen off from its own alpha CH group leading to an enol.
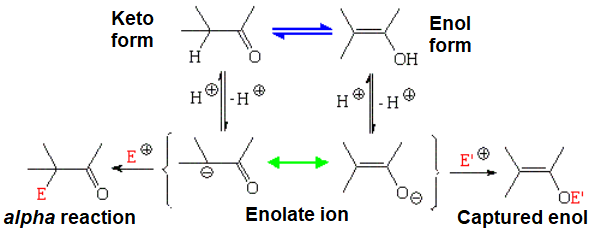
Cartoon of the keto-enol equilibrium
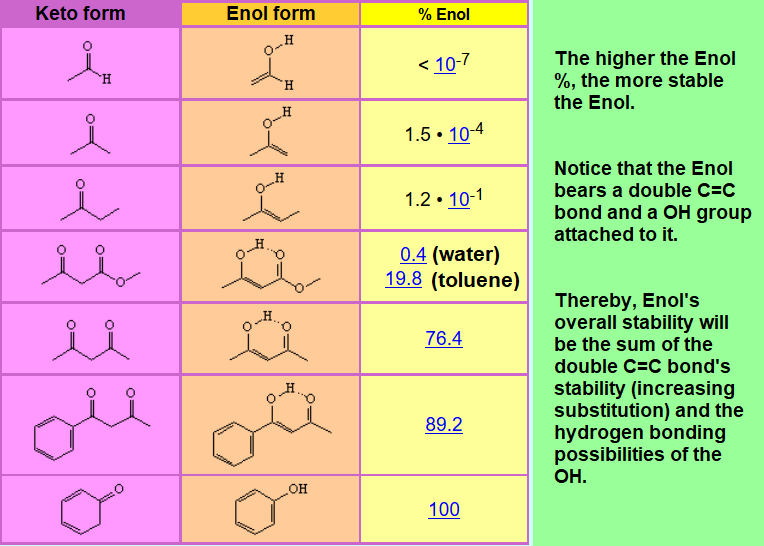
The keto-enol equilibrium is generally quite shifted to the keto form in "normal" aldehydes and ketones.
It's instructive if you analyze the experimental amount of enol found in different carbonyl compounds.
Would you be able to give explanations to the observed variations as a function of structure and in one case of the solvent?
Look in problems and exercises to get an answer.
THE ENOLIZATION OF ALDEHYDES AND KETONES HAS IMPORTANT CHEMICAL CONSEQUENCES
Please, notice that the keto and enol forms are different chemical species in equilibrium (double blue arrows; there's an atom that swaps places), while the enolate ion is a unique species that is described by two resonance forms (double-point green arrow; no atom swaps places).
The most common reaction of an enolate ion happens at the alpha carbon, importantly yielding a new C-C bond.
Nevertheless, some reactions take place at the oxygen allowing us to capture the enolate form. Remember this later on.