The aldol condensation is a typical reaction of aldehydes bearing hydrogens in alpha position relative to the carbonyl group.
Some ketones give this reaction as well.
In general, the aldol condensation is an equilibrium unfortunately shifted to the starting materials.
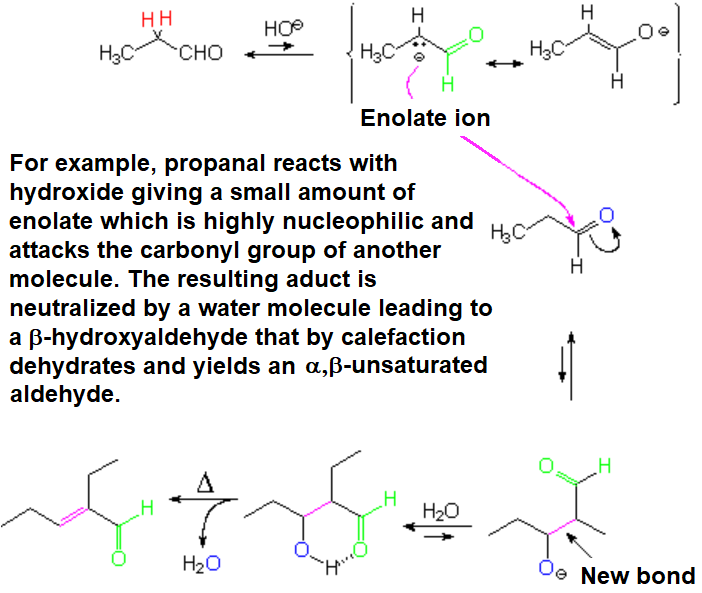
The aldol condensation leads to an ALDOL or beta-hydroxyaldehyde, in low ratio.
Nevertheless, the heating of the aldol compounds induces their dehydration yielding an alpha,beta-unsaturated aldehyde.
This water loss "pulls" and shifts the equilibria to the final product.
The self-condensation of an aldehyde with alpha hydrogens renders a new aldehyde, an alpha,beta-unsaturated one.
However, this reaction has some severe limitations...
FIRST LIMITATION
It was already mentioned: The equilibria of an aldol condensation are usually shifted to the starting material.
A way of shifting them to the products is the avoidance of continuous contact between the aldehyde and the base.
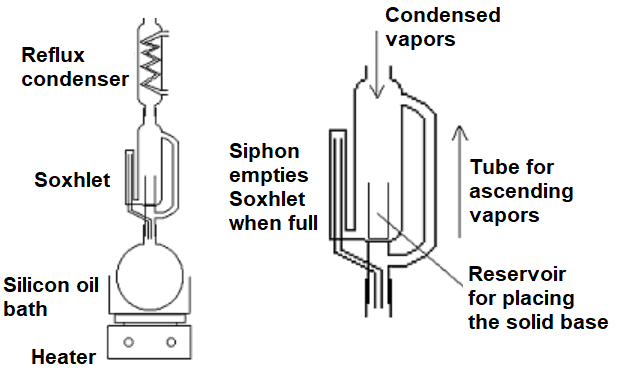
This can be achieved with the so-called Sohxlet aparatus.
SOLUTION TO THE FIRST LIMITATION
The aldehyde or ketone placed in the round-bottomed flask boils and, after returning to the liquid state at the cooled condenser, it accumulates in the Sohxlet reservoir where it gets in temporary contact with the base. The reaction proceeds there to the extent ruled by the equilibrium.
When the Sohxlet is full, the siphon empties it and the liquid in equilibrium returns to the flask. The small amount of formed aldol stops being in contact with the base - thus avoiding its going back to the starting material, and remains at the flask because its boiling point is much higher than that of the starting material.
The cycle repeats all over and over again (overnight for example) and the liquid at the flask ends up highly enriched in the pursued aldol.
SECOND LIMITATION
Generally speaking, the aldol condensation cannot be performed between different aldehydes or ketones (cross aldol condensation) because the result would be a tangled mixture:
Cross Aldol Condensation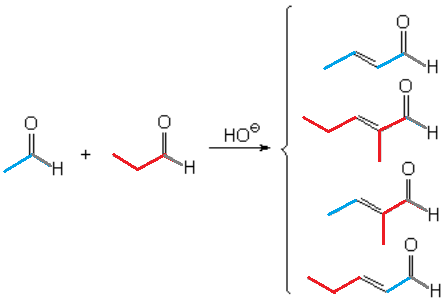
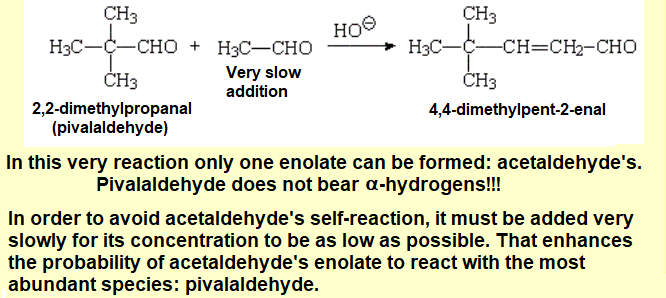
For example, acetaldehyde and propionaldehyde would yield four very alike products in similar ratios.
The enolate ions of both aldehydes would be produced at similar rates and their chances to attack molecules of either aldehyde would be identical.
A SOLUTION TO THE SECOND LIMITATION
If for one, a reactant DOES NOT bear alpha hydrogens, i.e. like pivalaldehyde or benzaldehyde, and for two, the other aldehyde is slowly added to the mixture, the cross aldol condensation may be achieved in high yield.
ANOTHER SOLUTION TO THE SECOND LIMITATION
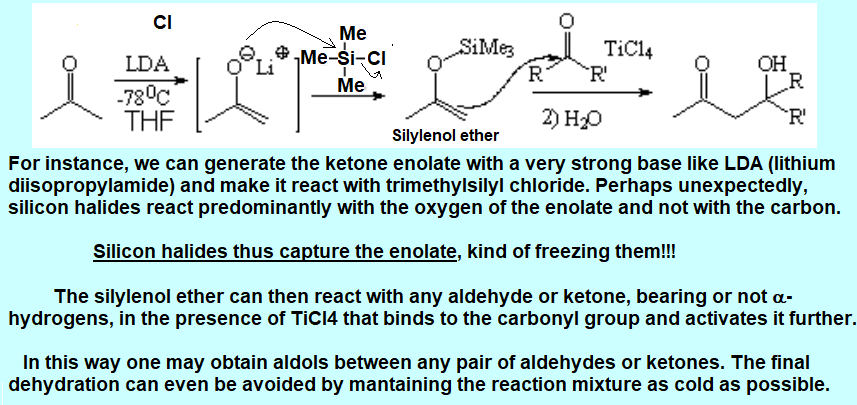
Use enamines or...
enolate trapping with a silicon derivative:
IMPORTANT
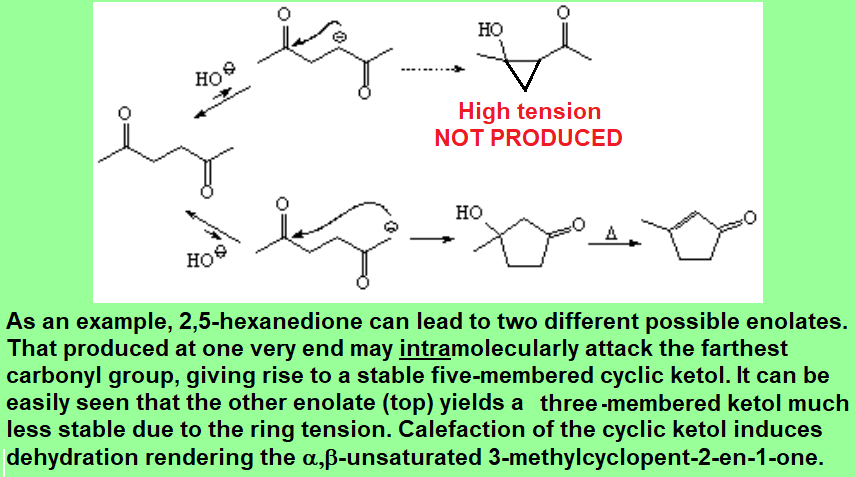
Aldol condensation can be performed in an intramolecular fashion, with the proper dialdehydes or diketones.
That allows us to get highly functionalized five- or six-membered cyclic compounds.