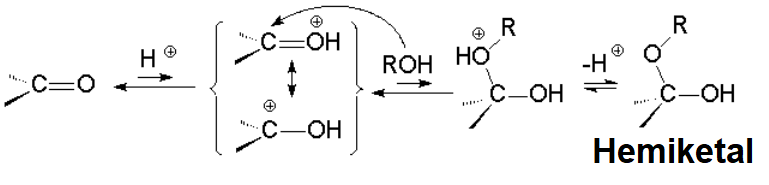
ALCOHOL ADDITION TO ALDEHYDES AND KETONES: HEMIKETAL FORMATION
The reaction follows suit the hydrate formation, save for the reactant that now is an alcohol instead of water.
Hemiketals ("half a ketal") are relatively unstable and the equilibria are usually shifted to the starting materials.
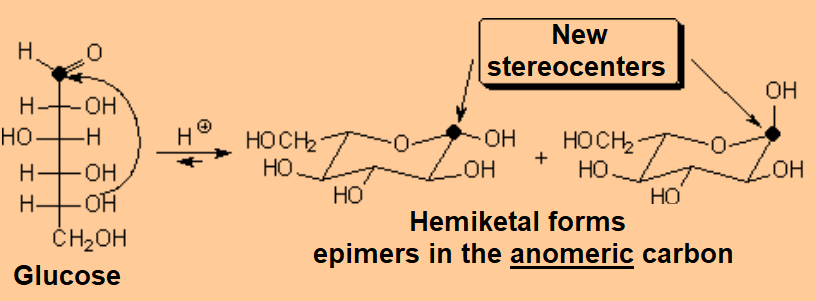
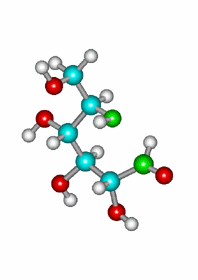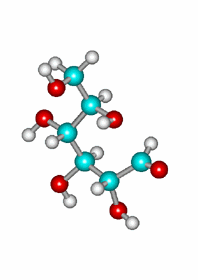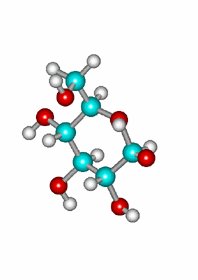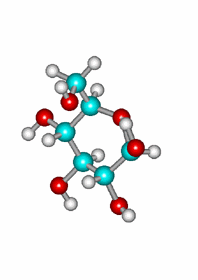
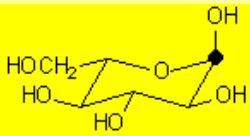
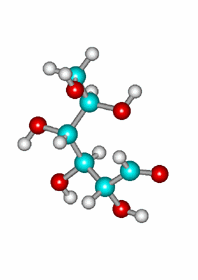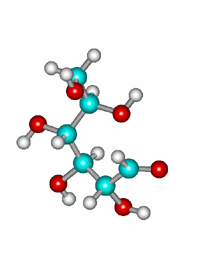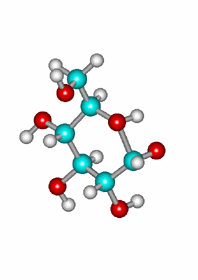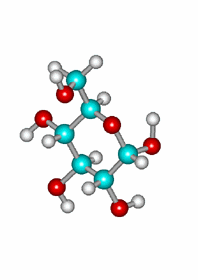
A very important exception is the intramolecular reaction in carbohydrates (sugars) where the resulting cyclic hemiketals are very stable.
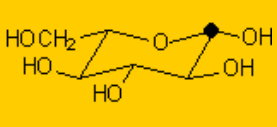
One will never find sugars like glucose as open chain compounds but as five- or six-membered rings, by means of the intramolecular formation of hemiketals.
The attack of the OH group in 5 position to the carbonyl by its upper or lower faces leads to a pair of diastereomers in different ratio.
Attack of the OH(5) to one of the carbonyl's faces leading to the axial epimer.
Attack of the OH(5) to the other carbonyl's face leading to the equatorial epimer.
Since the reaction does not involve any configuration change of glucose's stereocenters and a new one is created on the formerly carbonyl's carbon, a mixture of two diastereomers is formed whose physical and chemical properties are different.