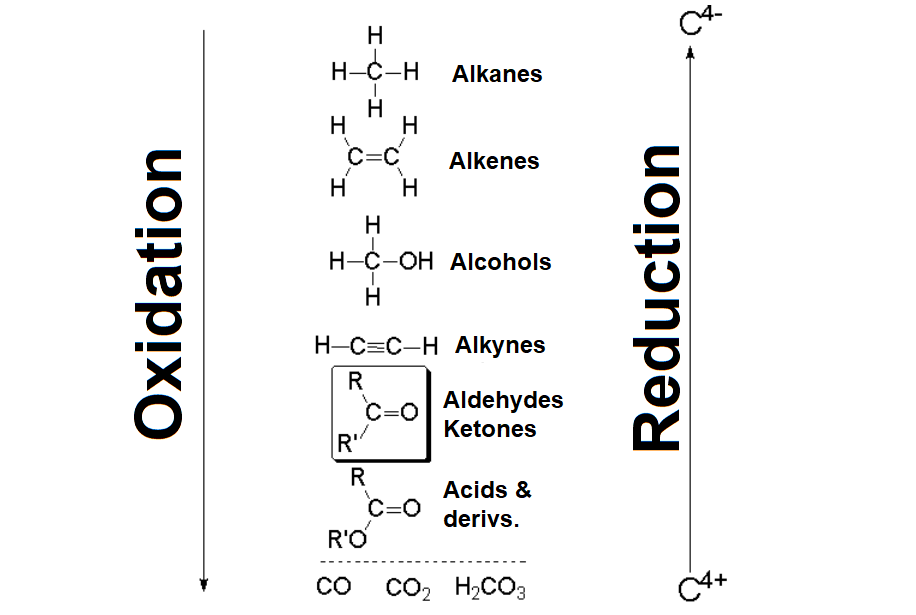
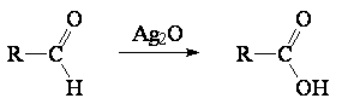OXIDATION OF ALDEHYDES AND KETONES
In aldehydes and ketones, the formal oxidation state of the carbonyl carbon is +1 and +2, respectively.
It is +3 in carboxylic acids meaning that an aldehyde or ketone still has a chance to oxidize further on.
Aldehydes or ketones render carboxylic acids with the appropriate oxidant.
It is fairly easy and can be achieved with very smooth oxidants like silver oxide (Ag2O).
Silver oxide is a very smooth oxidant indeed that is quite selective to aldehydes.
Any other function WON'T be oxidized by Ag2O.
Of course, stronger oxidants (KMnO4, K2Cr2O7) that do oxidize other functions, will oxidize aldehydes as well to carboxylic acids.
IMPORTANT: If there is an aldehyde in a complex molecule and one does not want it to be oxidized, "protection" is the answer.
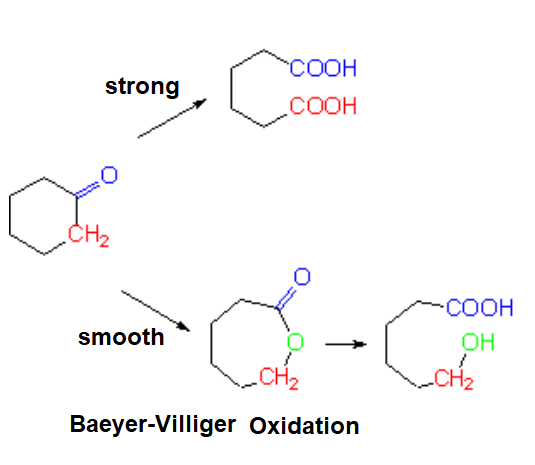
Ketone oxidation implies the rupture of a C-C bond.
If it is energic (KMnO4, K2Cr2O7) two carboxylic groups will be produced.
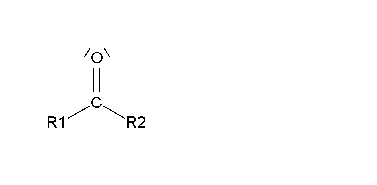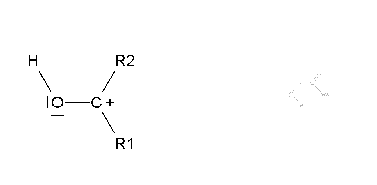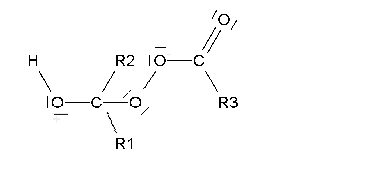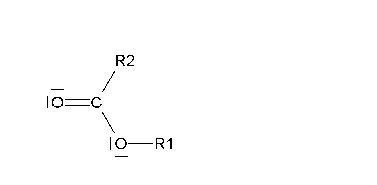
If it is smooth (Baeyer-Villiger oxidation), an ester is produced that, once it is hydrolized, gives rise to a carboxylic acid and an alcohol.
If the ketone is not cyclic, its oxidation leads to a mixture of two separate carboxylic acid molecules which is quite inconvenient from a synthetic point of view.
However, the oxidation of a ketone group contained in a cycle is exempt of that inconvienience for obvious reasons.
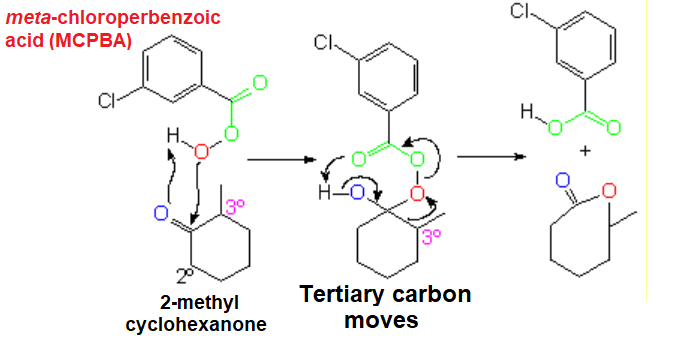
Baeyer-Villiger Oxidation
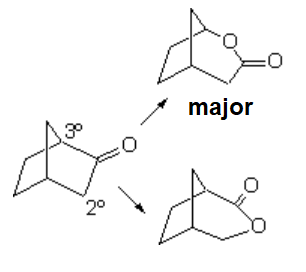
The smooth oxidation of non-symmetric ketones poses the problem of which side the oxygen would insert in.
The reaction is usually rather regioselective, the oxygen inserting in the side of the most substituted carbon.
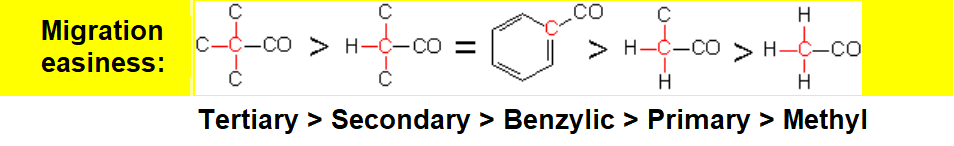
Oxygen insertion (red) involves the migration of one of the alkyl groups. The higher its substitution, the larger its migration chances.