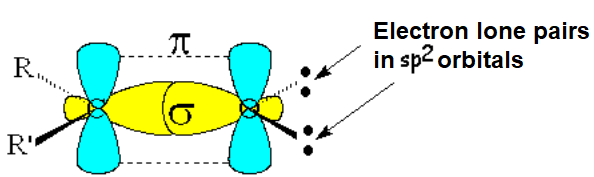
Like a double C=C bond, the carbonyl group C=O is formed from two molecular orbitals, one sigma and another pi.
The oxygen cannot bond to another atom and bears a couple of electron lone pairs in sp2 orbitals.
The experimentally measured bond angles and distances are compatible with a sp2 hybridization of the two atoms forming the C=O bond.
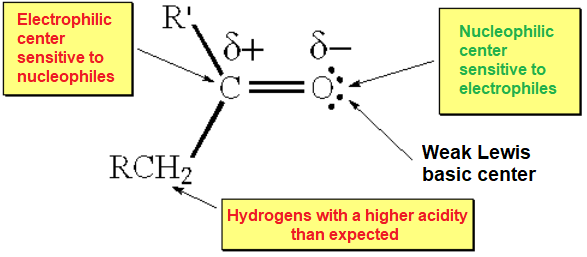
The charge-saparated resonance form explains the electron defficiency that carbon abides in a double C=O bond. This is an important difference with the C=C counterpart.
Oxygen's higher electronegativity induces a strong polarization of the C=O bond that marks its reactivity and, very IMPORTANT, that of its adjacent position (alpha).
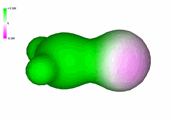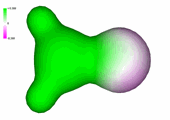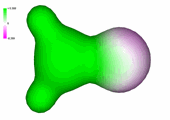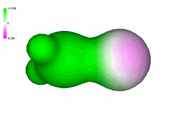 Charge density of formaldehyde
Charge density of formaldehyde
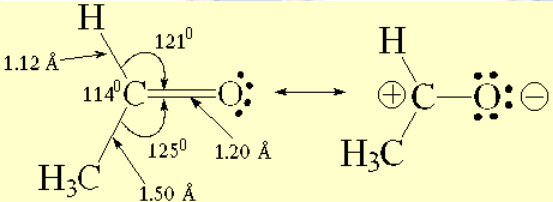 Structural features of acetaldehyde
Structural features of acetaldehyde