Two - Electron
Closed Shell
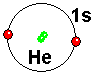
Helium has two electrons in the s orbital of shell 1. This electronic situation is so stable that Helium does not combine with any atom.
He has two electrons in the 1s atomic orbital. It has layer 1 "closed".
Hydrogen, which only has one electron, can acquire the configuration of Helium, which is very stable, by sharing its electron, plus another from another atom.
This is how a chemical bond is established: by the sharing of two electrons.
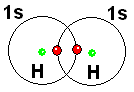
By sharing their electrons, each of the two hydrogen atoms, at least at some point, possesses both electrons.
The electrons of the hydrogen molecule (H2) now circulate around the two nuclei of H. Isn't the situation very similar to that of the He atom, which is so stable?
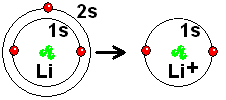
Lithium has two electrons in the s orbital of shell 1 and another in the s orbital of shell 2. If it loses the outermost electron, that of the 2s orbital, it is left in a situation very similar to that of He , which is especially stable. It is very easy for Li to completely give up an electron to another atom that is easy to accept it. Lithium forms bonds by giving up electrons, NOT by sharing them.
Lithium achieves a situation very similar to that of He by completely giving up an electron. But to what element will it give it up?
The electron is transferred to another element with high electron affinity, for example oxygen, fluorine, chlorine, bromine...
By giving up an electron, the Li atom retains the three protons in the nucleus. Therefore it becomes a positive ion (three protons compared to two electrons) whose size is much smaller than that of the starting atom: The three protons attract two electrons more strongly than does to three!
Hydrogen and Lithium, through two different methods of forming bonds with other atoms (sharing and electronic transfer, respectively) manage to obtain, when forming a molecule, the configuration of Helium that has the complete or "closed" electronic layer 1.
Could you predict what will happen to the element Beryllium?