Neon (1s2 2s2 2p6) has two electrons in the s orbital of shell 1, two electrons in the s orbital of shell 2 and six electrons in the p orbital of shell 2. This electronic situation is so stable that Neon does not combine with any atom.
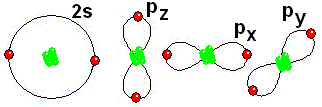 IMPORTANT: Shell 1 orbitals have been omitted. The shell 2 orbitals have been separated to give more clarity to the drawing. They would all be superimposed, around the 10-proton nucleus.
IMPORTANT: Shell 1 orbitals have been omitted. The shell 2 orbitals have been separated to give more clarity to the drawing. They would all be superimposed, around the 10-proton nucleus.
Atoms such as Boron, Carbon, Nitrogen and Oxygen, which lack electrons to acquire the Neon configuration, achieve it by sharing electrons with other atoms.
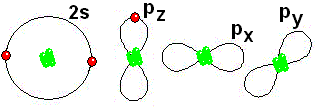 IMPORTANT: Shell 1 orbitals have been omitted. The shell 2 orbitals have been separated to give more clarity to the drawing. They would all be superimposed, around the 10-proton nucleus.
IMPORTANT: Shell 1 orbitals have been omitted. The shell 2 orbitals have been separated to give more clarity to the drawing. They would all be superimposed, around the 10-proton nucleus.
 This is how it gets closer to its "goal" of having eight electrons around it, like Neon
This is how it gets closer to its "goal" of having eight electrons around it, like Neon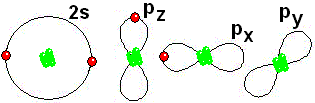 IMPORTANT: Shell 1 orbitals have been omitted. The shell 2 orbitals have been separated to give more clarity to the drawing. They would all be superimposed, around the 10-proton nucleus.
IMPORTANT: Shell 1 orbitals have been omitted. The shell 2 orbitals have been separated to give more clarity to the drawing. They would all be superimposed, around the 10-proton nucleus. To study the bonds between atoms, it is only interesting to consider the electrons in the outermost shell.
To study the bonds between atoms, it is only interesting to consider the electrons in the outermost shell.
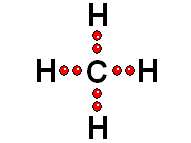 Thus it achieves the "goal" of having eight more outer electrons around it.
Thus it achieves the "goal" of having eight more outer electrons around it.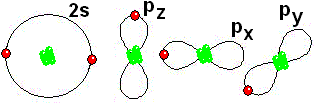 IMPORTANT: Shell 1 orbitals have been omitted. The shell 2 orbitals have been separated to give more clarity to the drawing. They would all be superimposed, around the 10-proton nucleus.
IMPORTANT: Shell 1 orbitals have been omitted. The shell 2 orbitals have been separated to give more clarity to the drawing. They would all be superimposed, around the 10-proton nucleus. To study the bonds between atoms, it is only interesting to consider the electrons in the outermost shell.
To study the bonds between atoms, it is only interesting to consider the electrons in the outermost shell.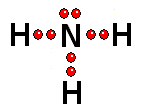 Thus it achieves the "goal" of having eight outermost electrons around it, leaving two unshared electrons.
Thus it achieves the "goal" of having eight outermost electrons around it, leaving two unshared electrons.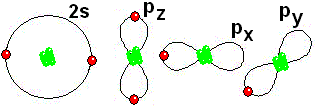 IMPORTANT: Shell 1 orbitals have been omitted. The shell 2 orbitals have been separated to give more clarity to the drawing. They would all be superimposed, around the 10-proton nucleus.
IMPORTANT: Shell 1 orbitals have been omitted. The shell 2 orbitals have been separated to give more clarity to the drawing. They would all be superimposed, around the 10-proton nucleus.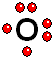 To study the bonds between atoms, it is only interesting to consider the electrons in the outermost shell.
To study the bonds between atoms, it is only interesting to consider the electrons in the outermost shell.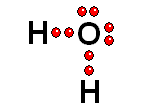 Thus it achieves the "goal" of having eight outermost electrons around it, leaving two pairs of unshared electrons.
Thus it achieves the "goal" of having eight outermost electrons around it, leaving two pairs of unshared electrons.| BH3 | CH4 | NH3 | H2O |
Sodium and Magnesium have complete electron shells 1 and 2 and one and two electrons, respectively, in the s orbital of shell 3. It is very easy for them to completely give up one electron (Sodium) or two (Magnesium) to another atom that be easy to accept them. Sodium and Magnesium form bonds by giving up electrons, NOT by sharing.
For its part, Fluorine lacks one electron to have complete shell 2.
It is very easy for it to accept an electron from another atom that gives it up easily to achieve the Neon configuration.
Fluorine forms bonds by accepting electrons.