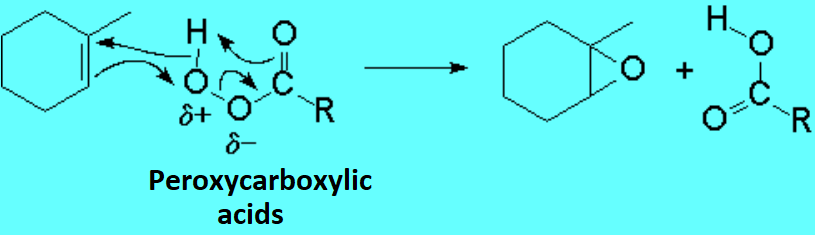
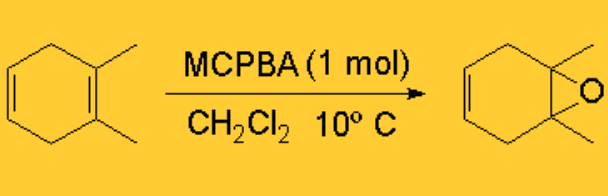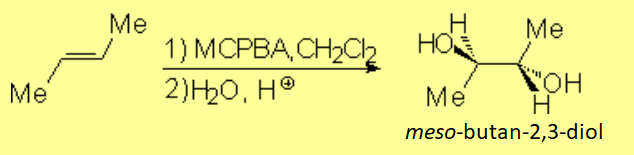Oxidation of olefins with peroxyacids leads to epoxides in a direct, easy and clean manner.
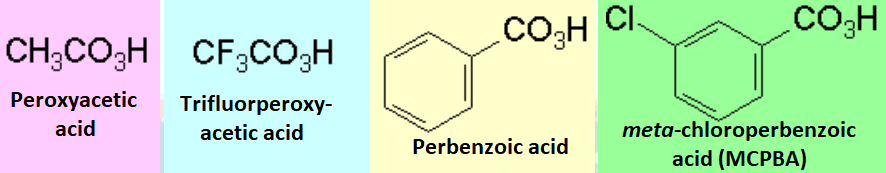
The most utilized peroxycarboxylic acids are:
The reaction is very selective:
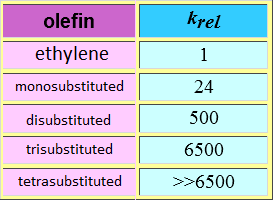
The peroxyacid is attacked by the olefin bearing the highest electronic density, i.e. by the most substituted one.
Olefin epoxidation combined with the aperture of the resulting epoxide with water in acidic medium, is an excellent method to get anti-alcohols:
The trans stereochemistry of the starting olefin makes the reaction produce the meso form.
In turn, the cis olefin leads to a racemic mixture.