PREPARATION OF ALCOHOLS BY NUCLEOPHILIC SUBSTITUTION
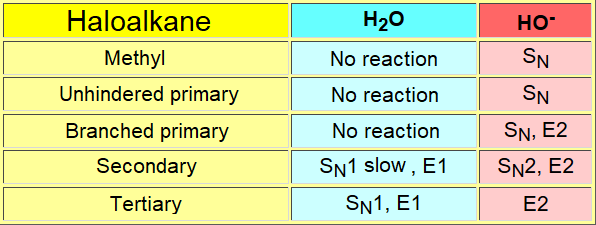
One can perform the substitution of a good leaving group by reaction with water (hydrolysis) or with hydroxide as, respectively, weak and strong nucleophiles.
The hydroxide ion is a more powerful nucleophile - and a strong base! - than water and the substitution can end up very differently depending on the structure of the starting material.
Please, consider that this method is not in general very useful to get alcohols.
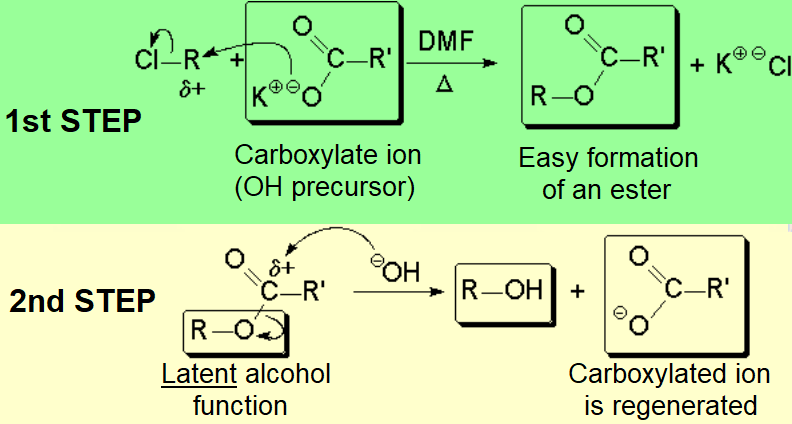
An important alternative is the performing of the substitution with a precursor function of the hydroxyl group.
The OH is maintained LATENT along the first step of the reaction to be finally released in the second step.
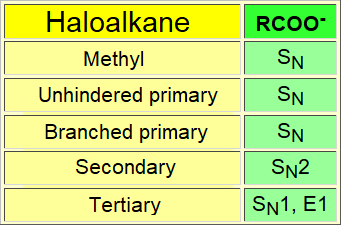
The carboxylate ion is a very good nucleophile but a very weak base.
The substitution reaction proceeds with much better results using this trick!!!
The much lower basicity of the carboxylate nucleophile prevents the competion of the elimination reaction.
Let me insist!
The lower basicity of the nucleophile avoids the usually hard competion of the elimination that otherwise would take over.
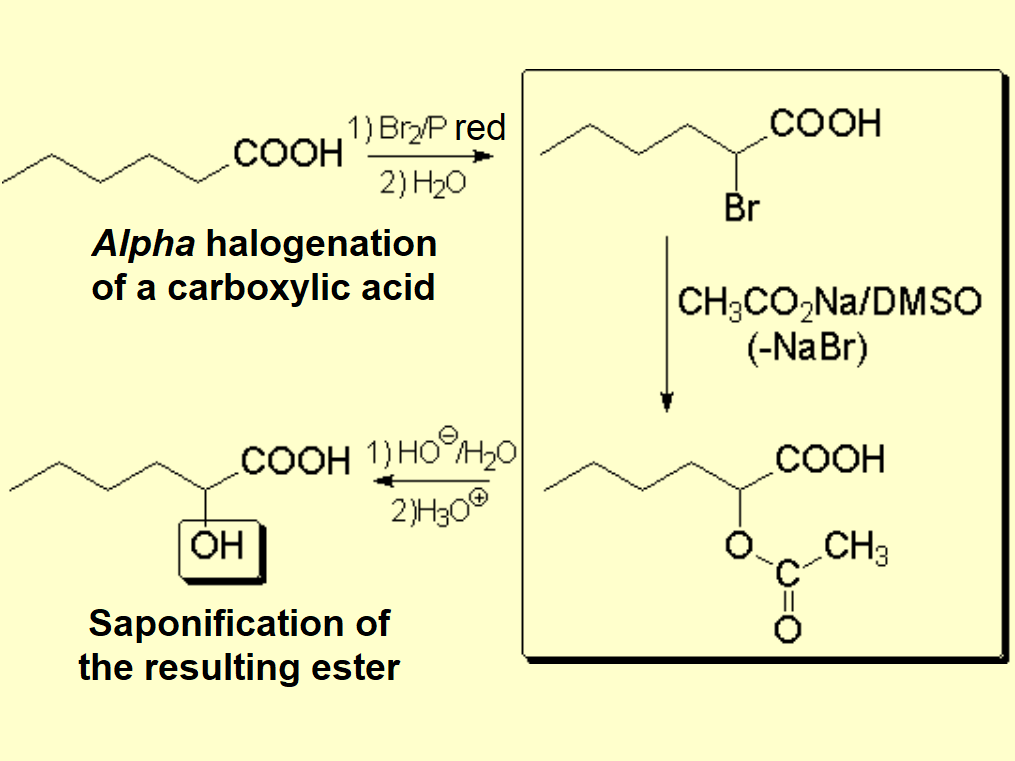
Look at this practical example: