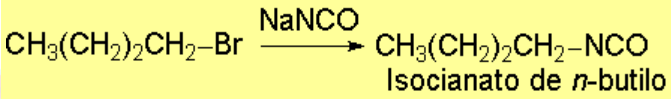
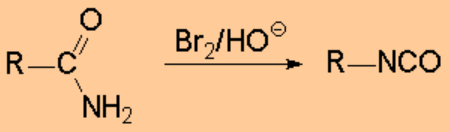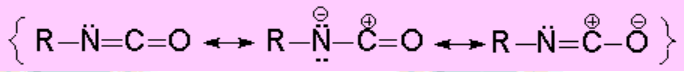The isocyanides can be prepared by nucleophilic substitution with CYANATE. This ion is a very good nucleophile and a weak base and therefore it leads to typical SN2 reactions, unless the reacting carbon is too sterically hindered.
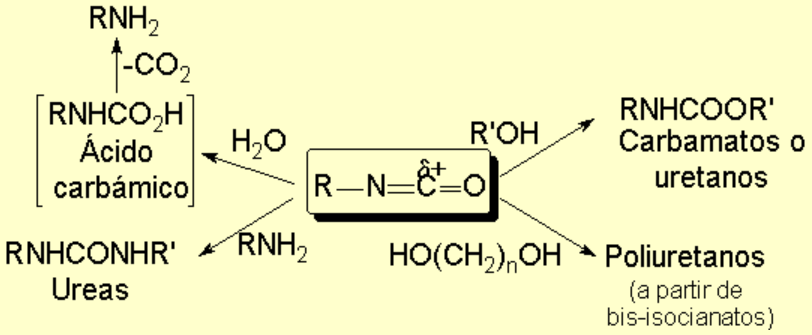
Due to that feature, the main reactions of isocyanides imply the nucleophilic attack on that carbon, yielding a large variety of new functional groups: