For instance, formiate ion is completely symmetrical.
The C-O bond lengths are identical and the negative charge is equally shared between the two oxygens.
Overlapping of the p orbitals of the O-C-O fragment of formiate, showing the extreme electron delocalization.
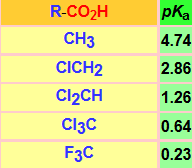
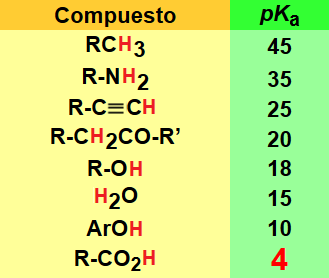
The acidity of a carboxylic acid is biased by the structure of the R group (Table).
An electron-releasing R group will destabilize the carboxylate anion and make the acid less acidic. This is the case of the alkyl R groups.
In contrast, an electron-withdrawing R group will stabilize the carboxylate anion even further, thus making the acid more acidic. This is the case of the halo-, ammonium- and nitromethyl groups.
The increment in the number of halogen atoms increasingly stabilizes the carboxylate anion and the acidity goes up (pKa down).
The change of chlorine by fluorine, more electronegative, increases the acidity.
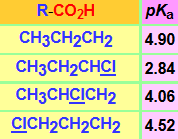
The presence of a chlorine atom in the neighbouring position to the carboxylate group delocalizes the negative charge and augments the acidity of the conjugate acid.
But this effect is very sensitive to distance, isn't it?
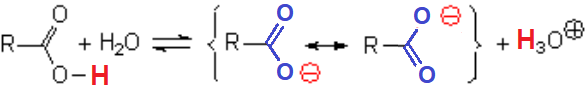 Carboxylate anion's resonance forms are completely equivalent and the negative charge is located on the oxygen atoms, very electronegative ones.
Carboxylate anion's resonance forms are completely equivalent and the negative charge is located on the oxygen atoms, very electronegative ones.