FORMATION OF ACID (ACYL) HALIDES
The transformation of a carboxylic acid into an acid (acyl) halide renders a much more reactive compound in its C=O group due to the presence of the halogen atom.
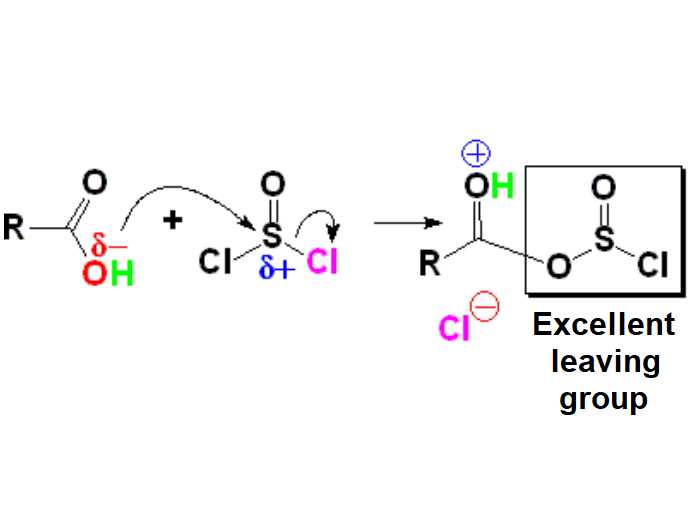
The reaction is usually performed with thionyl chloride, reagent already used to substitute OH groups by Cl in alcohols.
The sulfur atom of thionyl chloride is strongly electrophilic because is bonded to three very electronegative atoms, thus behaving as a strong Lewis acid, that can be attacked by the OH of an alcohol or a carboxylic acid. A molecule of HCl is expelled in the process.
A kind of organic-inorganic anhydride is then formed that is immediately protonated by the expelled HCl.
This intermediate is well prepared for chloride attack since it contains an excellent leaving group and the protonation strongly enhances its C=O carbon electrophilicity.
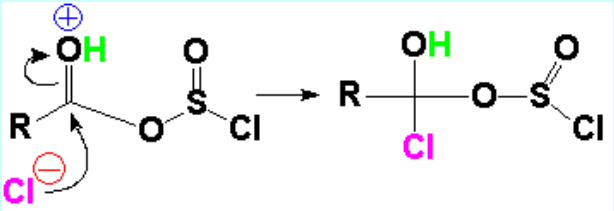
At this stage chloride attacks the C=O group.
This is the addition step where the former C=O carbon goes from sp2 to a sp3 tetrahedral intermediate.
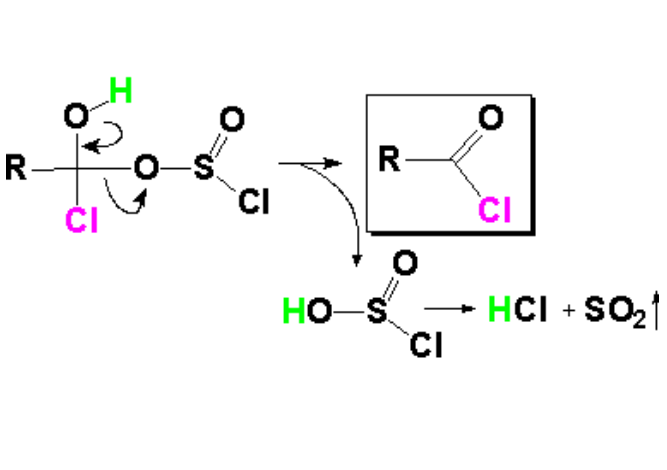
In the last elimination process, the leaving group created at the first step leaves.
The release of SO2 gas shifts the various equilibria to the formation of the acid chloride.
As usually, the loss of the leaving group in the elimination step allows the carbon to recover the sp2 hybridization.
Acid (acyl) chlorides are very reactive compounds (very sensitive to moisture that must be avoided at all times) used as precursors of other functions derived from carboxylic acids.