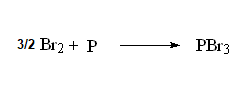ALPHA-HALOGENATION OF CARBOXYLIC ACIDS
The alpha-halogenation of carboxylic acids is achieved with red atomic phosphorous and an excess of molecular bromine.
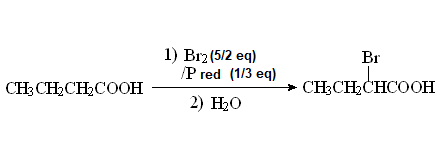 The reaction was named after its discoverers and developers: Hell-Volhard-Zelinski
The reaction was named after its discoverers and developers: Hell-Volhard-Zelinski
The alpha position to a carboxylic acid function is therefore activated in order to make the compound react further on.
Carl Magnus von Hell (8 September 1849 – 11 December 1926) was the German chemist who discovered, together with Jacob Volhard and the Russian chemist Nikolay Zelinsky, the alfa-halogenation reaction of carboxylic acids.
Jacob Volhard (4 June 1834 – 14 January 1910) was the German chemist responsible for the improvement of the Hell-Volhard-Zelinsky halogenation.
Nikolay Dimitrievich Zelinsky (6 February n.s., 1861 in Tiraspol, Russian Empire – 31 July 1953 in Moscow), Russian and Soviet chemist, academician of the Academy of Sciences of USSR (1929). Zelinsky studied at the University of Odessa and at the universities of Leipzig and Göttingen in Germany. Zelinsky was one of the founders of theory on organic catalysis. He is the inventor of the first effective filtering activated charcoal gas mask in the world (1915).
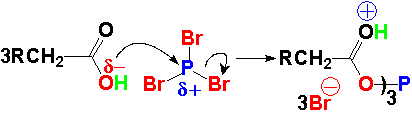
The central phosphorous of PBr3
is pretty electrophilic because is bonded to three very electronegative bromine atoms. It is thus easily attacked even by a weak base like carboxyl's oxygen.
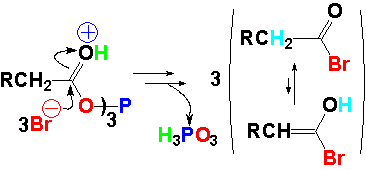
A triple anhydride is then formed. The phosphorous-bonded oxygen becomes an excellent leaving group. The by-product HBr protonates the C=O group and makes it ready for the bromide nucleophilic addition.
The nucleophilic bromide attack leads to an acid bromide.
The reaction is quite similar to that of thionyl chloride.
The acid bromide with alfa-hydrogens is in equilibrium with a few enol molecules.
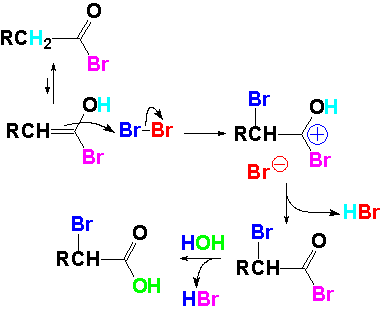
The bromide reaction produces an intermediate where the attacked carbon turns to sp3 hybridization. From that intermediate hypophosphorous acid is lost, the carbon recovering the sp2 status. The few enol molecules in equilibrium are able to split up the excess Br2 molecules.
Enol molecules break up the bromine molecules in excess.
A bromine atom is then added to the double bond ending up at the alpha position.
At this point, the reaction leads to an alpha-bromo acid bromide.
Its mild hydrolisis finally renders the alpha-bromo carboxylic acid.
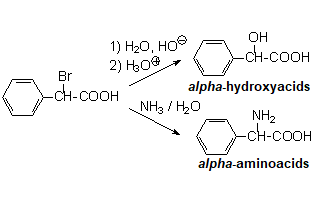
Alpha-halogenation of carboxylic acids is a very useful reaction that allows us to activate the neighboring position to a carboxylic acid function and obtain interesting products like alpha-hydroxy- and alpha-amino acids.
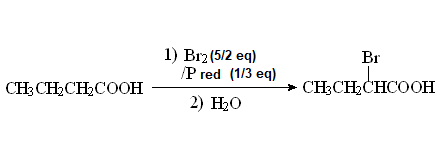 The reaction was named after its discoverers and developers: Hell-Volhard-Zelinski
The reaction was named after its discoverers and developers: Hell-Volhard-Zelinski