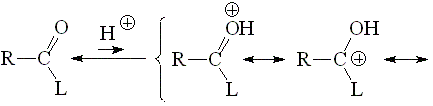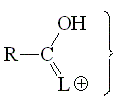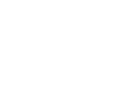ACID-BASE PROPERTIES OF AMIDES
Basicity of the Amide Group
The basicity of an amiDe (with "d") group can be understood from the acidity of its protonated form. The higher the acidity of the latter, the lower the basicity of the amide.
The higher the participation of the third resonance form, the more stable the protonated form of a carbonyl derivative and therefore the less acidic or the more basic.
In other words, the larger the L group's capability of releasing electrons, the higher the basicity of the carbonyl group.
Would you explain the following pKa values just applying the previous rationale?
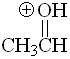 Aldehyde
Aldehyde
pKa = -8.0
The third resonance form is simply not possible when L = H. Therefore the protonated form is very unstable and aldehydes are the most acidic (the least basic) in the series.
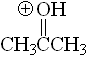 Ketone
Ketone
pKa = -7.2
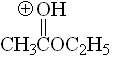 Ester
Ester
pKa = -6.5
The alkoxy group can stabilize the positive charge by mesomeric (resonance) effect. That explains why esters are even less acidic (more basic) than ketones.
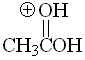 Acid
Acid
pKa = -6.0
The first and third resonance forms are equivalent for a protonated carboxylic acid. That makes up for an additional stabilization which leads to the apparent paradox that a protonated acid be the least acidic (the most basic) of the previous studied compounds.
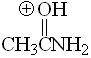 Amide
Amide
pKa = -0.4
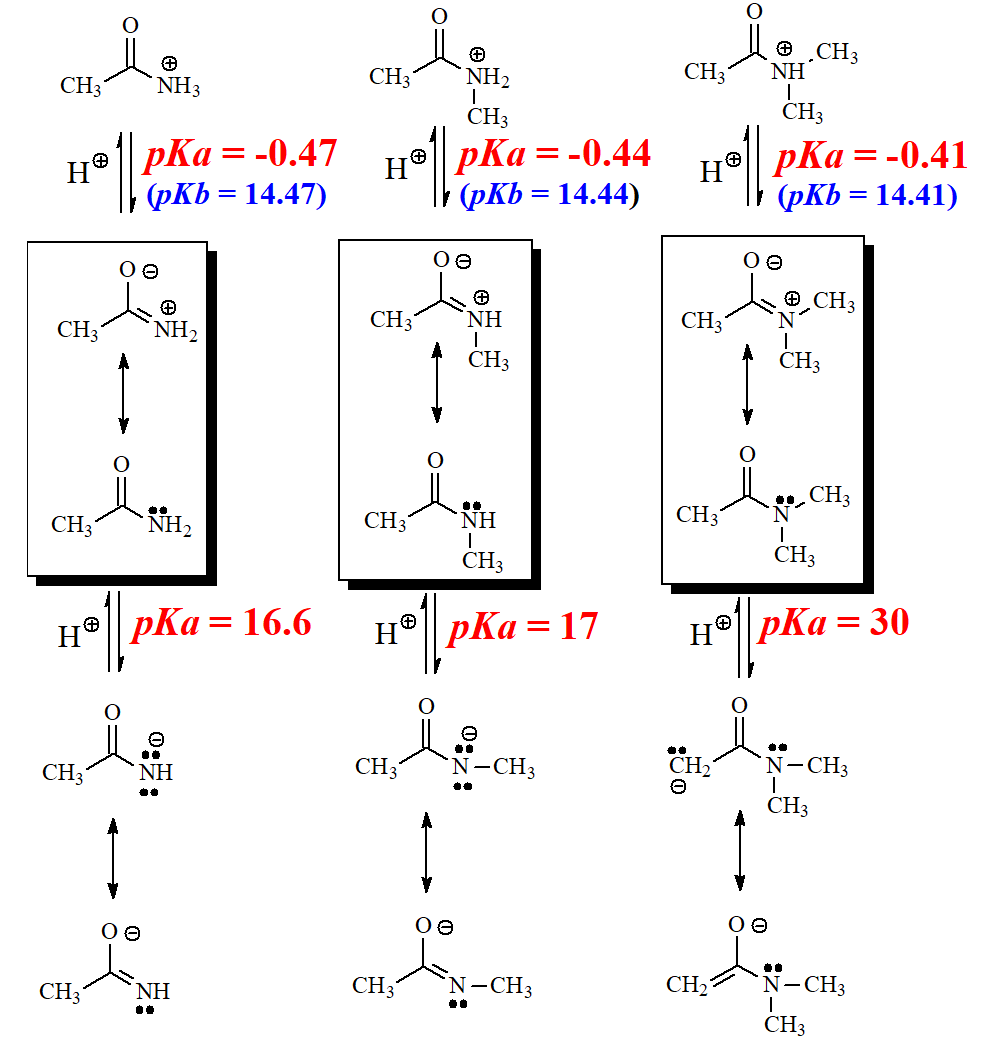
A protonated amide possesses the least acidic group of them all (CONH+), proof that the blinking resonance form has in this case an overwhelming contribution.
The electron lone pair on NH2 is very delocalized to the carbonyl group in amides.
Actually, the C=O group of an amide is more basic than the NH2.
That makes a huge difference in basicity between amiDes ("with d") and amiNes ("with N").
Acidity of alpha Hydrogens
In some amides, as for example acetamide or its N-methyl derivative, one can find two kinds of alpha hydrogens: those bonded to nitrogen and those bonded to carbon.
The hydrogens bonded to N are much more acidic than their C-bonded counterparts.
This is another manifestation of the increased electronegativity of N as compared to C.
This fact explains why acetamide and its N-methyl derivative display pKa = 17 whereas pKa = 30 for N,N-dimethyl acetamide that can only be deprotonated at C.