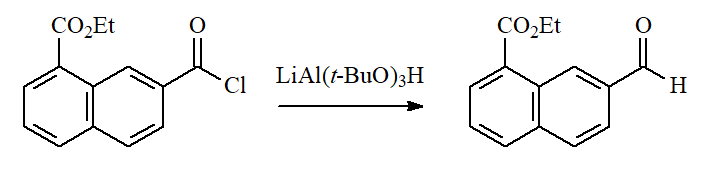REACTIVITY OF ACYL HALIDES
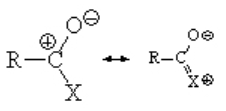
Acyl halides are the most reactive of all the carboxylic acid derivatives as explained by the negligible contribution of the resonance form with the positive charge on the L (halide) group.
The mechanism is the usual addition-elimination process.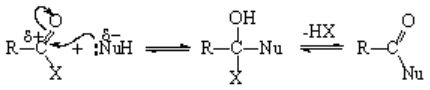
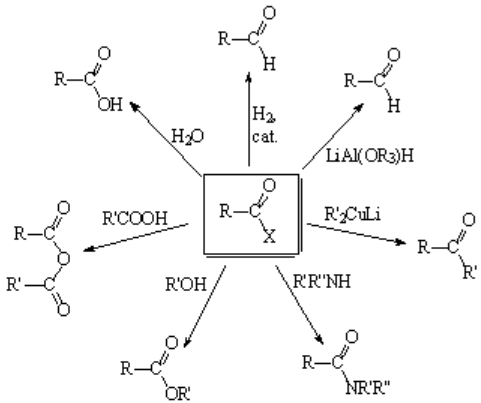
The versatility of the acyl chloride function is pretty wide allowing its transformation into other very important functional groups.
Some of them will be introduced to you for the first time...
Hydrolysis and Alcoholysis
Acyl halides can be converted into CARBOXYLIC ACIDS with the mere contact with moisture. Therefore, one has to be very careful and store acyl halides in a dry and inert atmosphere (dry nitrogen or argon).
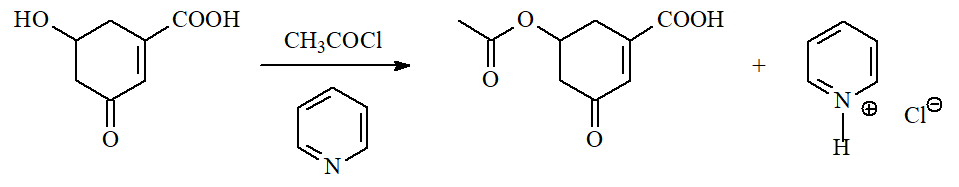
Acyl halides produce ESTERS with alcohols. The reaction does require a base in order to capture the released HCl.
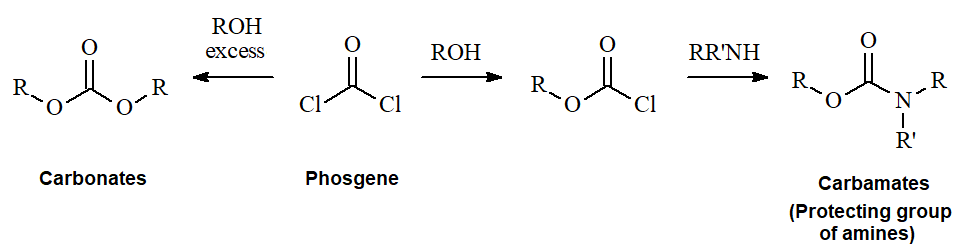
Phosgene is kind of "double" acyl halide from formic acid and can react with an alcohol excess to yield CARBONATES. If the amount of alcohol is controlled to just one equivalent, only one chloride is consumed and the remaining one can be made further react with amines rendering CARBAMATES.
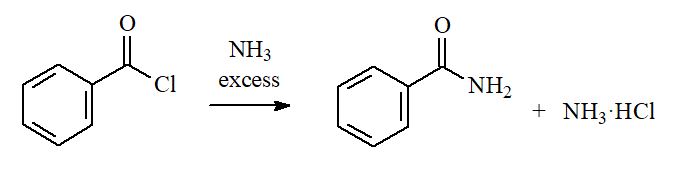
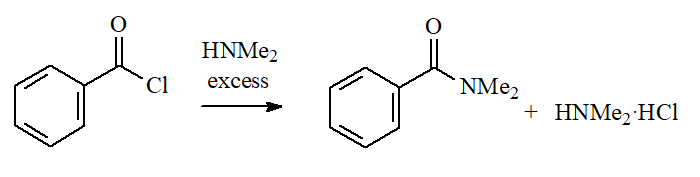
Ammonia and Amines
Ammonia and primery or secondary amines (NOT tertiary!) lead to AMIDES. The amine in excess is able to neutralize the released HCl.
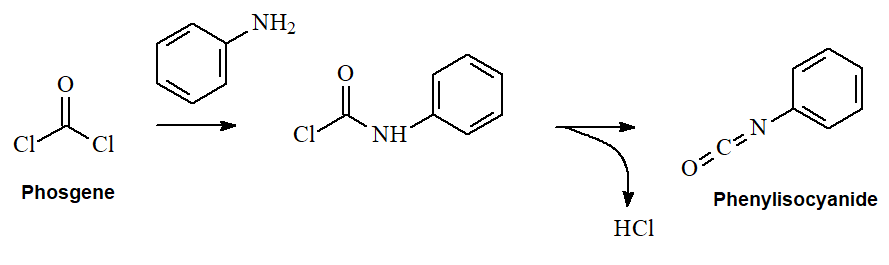
ISOCYANIDES (R-N=C=O) bear a functional group related to carboxylic acid derivatives. They can be prepared from phosgene and primary amines, going through an intermediate that possesses an acyl chloride Cl-C=O group.
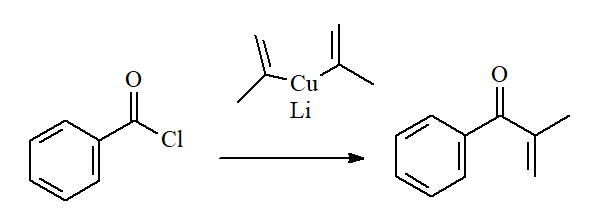
Covalent and low reactive organometallics render KETONES with acyl chlorides. An excellent example is the use of lithium alkylcuprates.
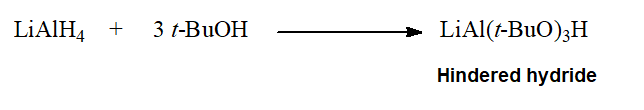
"Deactivated" hydrides are able to undergo the formal replacement of chloride by hydride on acyl chlorides leading to ALDEHYDES.
Many functional groups endure this smooth reduction conditions.
Why LiAlH4 cannot be used for the same purpose?
Hydrogenation with the Lindlar Catalyst
Do you remember Lindlar catalyst? Partially poisoned Pd with quinoline and other additives. The hydrogenation of acyl chlorides using this catalyst yields ALDEHYDES as well.
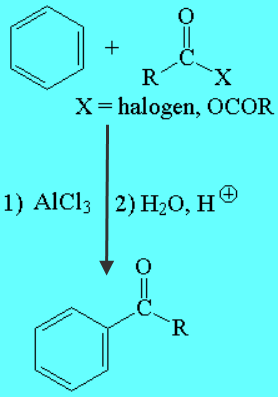
Aromatic Compounds (Friedel-Crafts Acylation)
Other Reactions (Ardnt-Eistert Reaction and Dehydrohalogenation)
Ardnt-Eistert reaction:
Diazomethane is able to react with acyl chlorides giving DIAZOKETONES, very reactive species that in weak oxidizing conditions rearrange yielding ESTERS.
The result of the reaction is the formal insertion of a CH2 group between the carbonyl and the R group formerly bonded to it. This process is named HOMOLOGATION of carboxylic acid derivatives.
Its mechanism is very complex but a very good excercise in the learning of Organic Chemistry.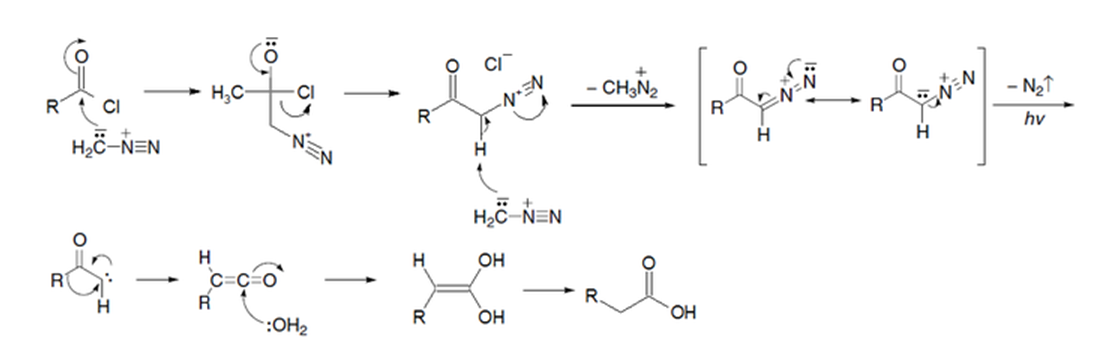
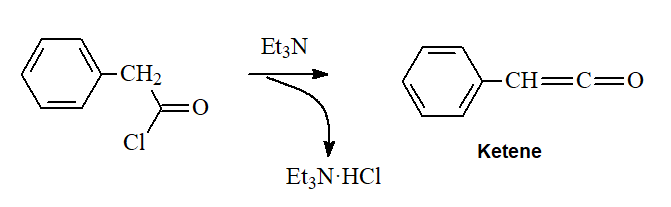
Tertiary amines CANNOT produce amides by reaction with acyl chlorides but their high basicity allow them to capture a molecule of HCl and transform acyl chlorides into KETENES (R-C=C=O).
IMPORTANT: For this reaction to proceed it is imprescindible that the acyl chloride have at least one alpha hydrogen.