NUCLEOPHILIC CONJUGATED ADDITION TO UNSATURATED ALDEHYDES AND KETONES
Besides the normal reactivity of C=C and C=O bonds, an alpha,beta-unsaturated aldehyde or ketone can undergo 1,4-reactions (conjugated or Michael addition).
This is similar to what happened in dienes, but there's a big difference:
In dienes the addition was electrophilic whereas in alpha,beta-unsaturated aldehydes or ketones the addition is electrophilic, due to the electron-withdrawing resonance effect of the C=O group.
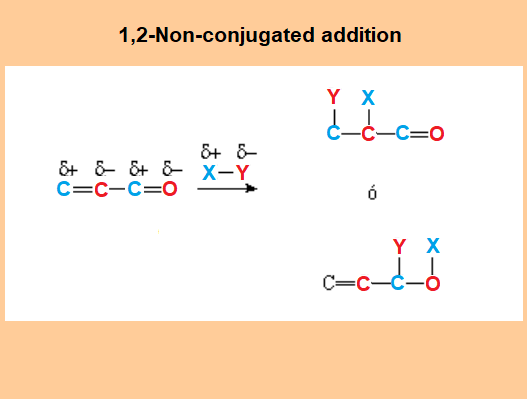
Non-conjugated Addition (1,2)
The NON-conjugated addition is effected on vicinal carbons (1,2) of the conjugated system.
There exist two possibilities:
Attack to C=C or to C=O.
In either case, the nucleophilic part of the reactant (Y) ends up bonded to either of the former two electron-defficient centers (blue).
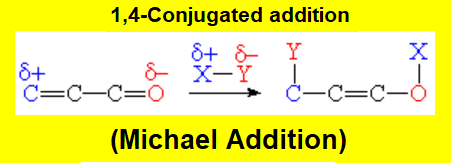
Conjugated Addition (1,4)
The conjugated addition happens at both ends (1,4) of the conjugated system.
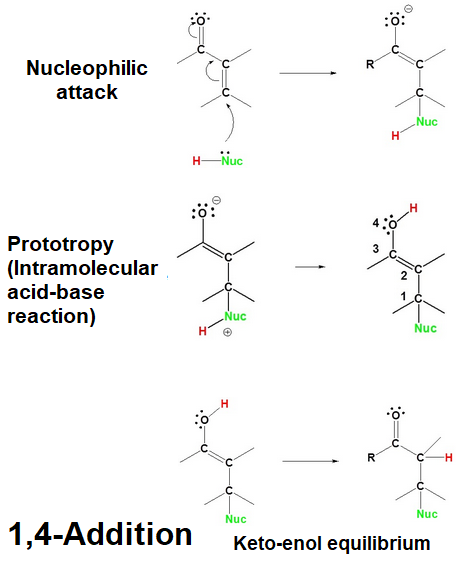
There's only one possibility and the nucleophilic part of the reactant (Nuc) attaches to the electron-defficient end of the conjugated atoms.
One double bond is maintained but it shifts to the central position.
When the reactant is Nuc-H, or a proton is added in a hydrolysis second step, an enol is produced whose equilibrium with the corresponding aldehyde or ketone is shifted to any of the latter two.
The reaction is highly regioselective:
It ALWAYS proceeds in 1,4 (conjugated) fashion.
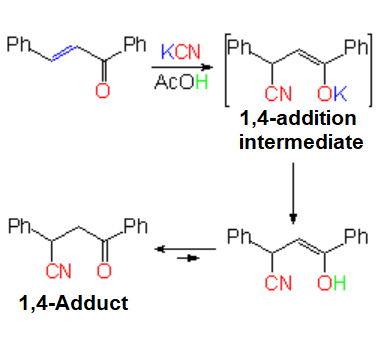
In cyanide addition, the nucleophilic part is the CN moiety
and the electrophilic one the potassium counterion.
An enolate is formed which is neutralized by acetic acid on the spot, leading to an enol in tautomeric equilibrium with the ketone.
The final result of the example is the 1,4-addition of cyanide to 1,3-diphenylpropenone.
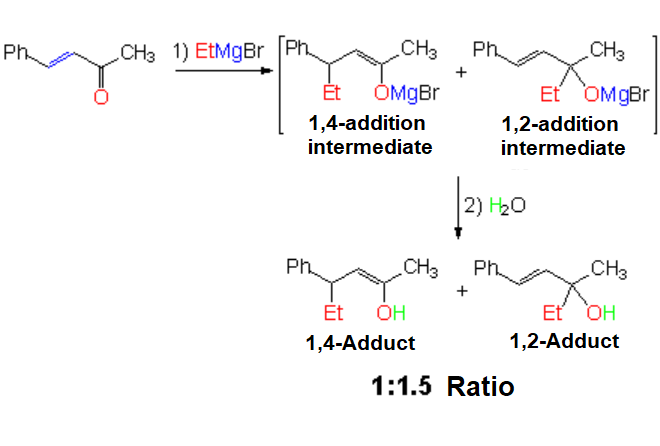
Grignard Reagent Addition (Organomagnesium Compounds)
The reaction COMPLETELY LACKS regioselectivity.
Grignard reagents are usually non-selective.
The example shows that they are like a bull in a china shop: ethylmagnesium bromide attacks the C=O group in all possible ways.
An inconvenient mixture is obtained.
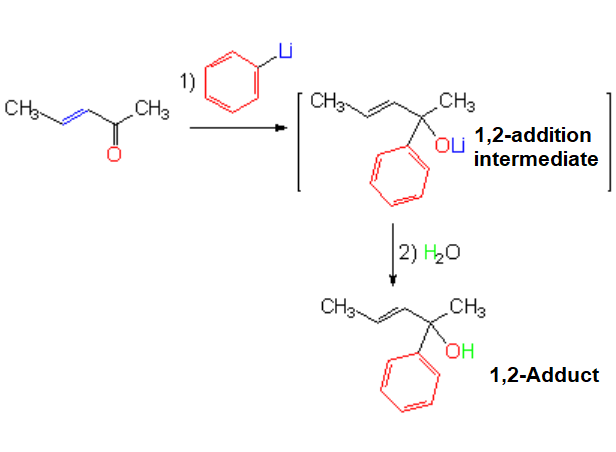
Addition of Organolithium Compounds
The reaction is regioselective...
YET, IT DOESN'T PROCEED 1,4 or conjugated.
Organolithium reagents are selective indeed but they act as if the double C=C bond wouldn't be there.
The are oblivious of the double C=C bond.
In the example, the phenyl group is the nucleophilic part and the metal is the electrophile.
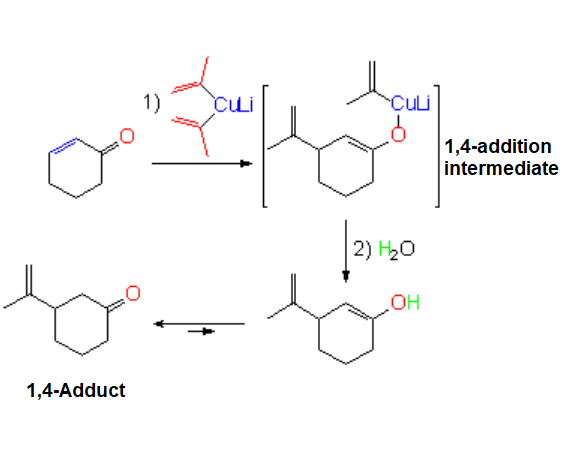
Addition of Lithium Organocuprates
The reaction is highly regioselective:
It ALWAYS proceeds in 1,4 (conjugated) fashion.
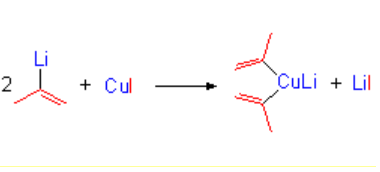
The organolithium compounds turn into organocopper derivatives in a straightforward manner, by reaction with cuprous iodide.
Organocopper compounds are organometallic reagents with a relatively large covalent character of the C-metal bond and therefore less reactive than the organolithium counterparts.
The carbon bonded to the Cu atom is the nucleophilic part that attacks 2-cyclohexenone in its electrophile part, i.e. the double C=C bond.
A new C-C bond is formed leading to an enolate whose reaction with water yields the enol and then the ketone by tautomeric equilibrium.
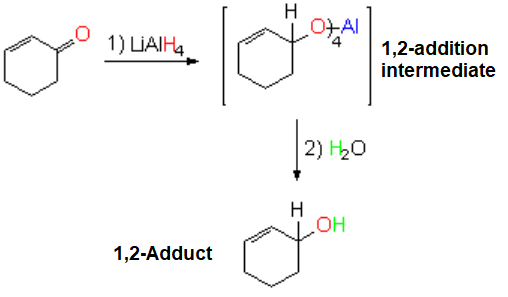
Hydride Addition (Lithium Aluminium Hydride, LAH)
The reaction is regioselective...
YET, IT DOESN'T PROCEED 1,4 or conjugated.
LAH is quite reactive and selective indeed and acts as if the double C=C bond wouldn't be there.
LAH ignores the existence of the double C=C bond.
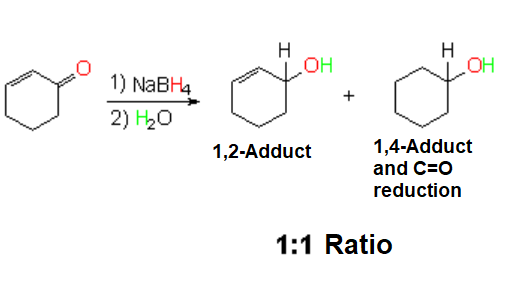
Hydride Addition (Sodium Borohydride)
The reaction COMPLETELY LACKS regioselectivity.
Sodium borohydride is actually way less reactive than LAH.
Paradoxically, it isn't selective at all.
Its use is not advisable with alpha,beta-unsaturated aldehydes or ketones because it leads to mixtures of reduced products difficult to separate.
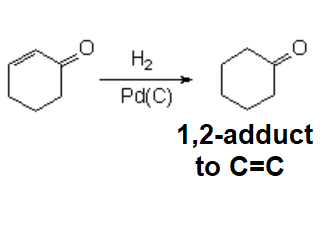
The reaction is regioselective...
YET, IT DOESN'T PROCEED 1,4 or conjugated.
Catalytic hydrogenation treats alpha,beta-unsaturated aldehydes or ketones as if the double C=C and C=O bonds would be isolated.
The double C=C bond reacts much faster in this reaction and gets reduced as shown in the example.