DIELS - ALDER CYCLOADDITION
The reaction between a conjugated diene and an olefin leads to a cyclohexene.
This is an amazing reaction in which two sigma C-C bonds are formed at the expense of two pi bonds.
The double C=C bonds of the diene cooperate, acting like tweezers, to attack the olefin.
For instance, 1,3-butadiene reacts with propenoic acid to yield 2-methylcyclohex-3-ene carboxylic acid.
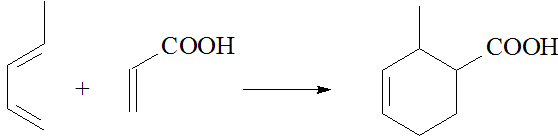
This reaction was discovered by the German organic chemists Diels and Alder who were awarded with the Nobel Prize in 1950.
Otto P. H. Diels (1876-1954): German chemist, Kiel University's professor, co-author together with Kurt Alder of the "Diene Synthesis", a procedure to obtain alkenes. In addition to the theoretical interest of these reactions, the method allowed for the synthesis of many molecules like camphor, vitamine D and cortisone. Nobel Prize of Chemistry shared with Alder in 1950.
Kurt Alder (1902-1958): German chemist, Colonia University's professor. Disciple of Otto Diels, he colaborated in Diels' research. They shared the Nobel Prize of Chemistry in 1950.
The reaction proceeds by a single TS in which the pi clouds of diene and olefin interact.
Three pi systems break up giving rise to two sigma bonds and a new pi system.
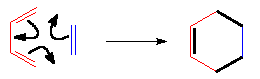 In the single TS the pi electrons circulate leading to the new bonds (in black).
In the single TS the pi electrons circulate leading to the new bonds (in black).
The resulting cyclohexene has been colored for you to easily identify which carbon comes from which molecule.
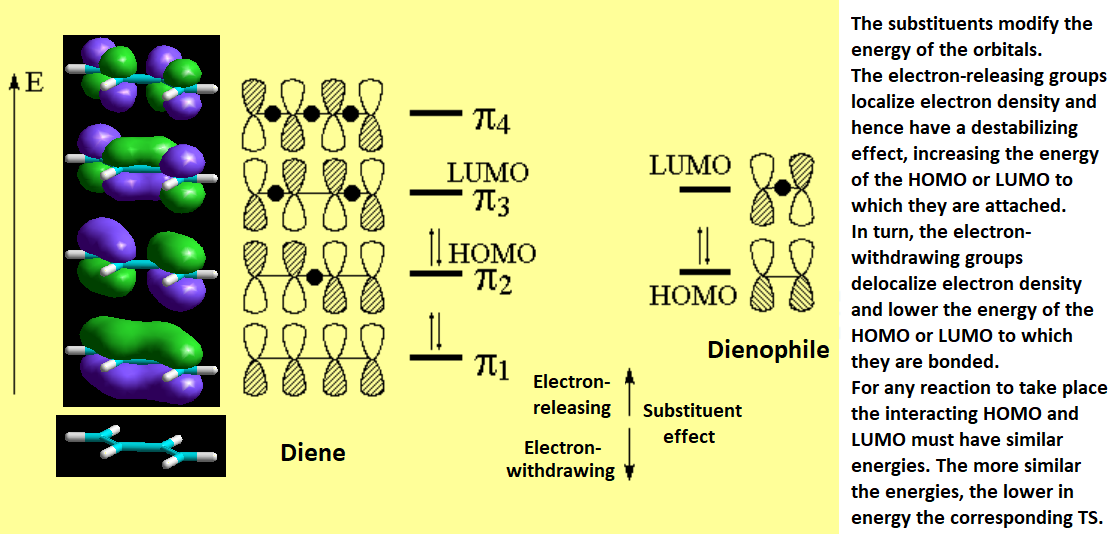
The interaction of the pi clouds in the TS can be easily understood by means of a simple molecular orbital diagram.
The Highest Occupied Molecular Orbital (HOMO) of one species has to interact (donate electrons) with the Lowest Unoccuppied Molecular Orbital (LUMO) of the other species: "The Nucleophile attacks the electrophile", as always.
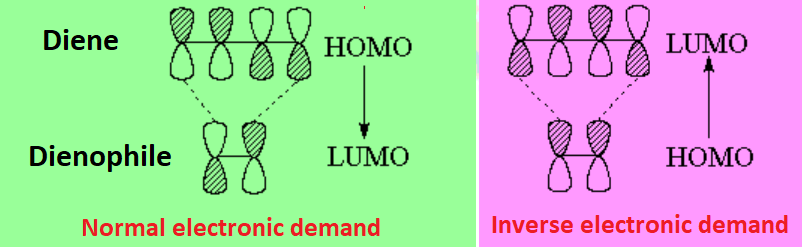
One can then have two situations. Diene's HOMO attacks olefin's LUMO or Diene's LUMO is attacked by olefin's HOMO.
Please, be aware that the symmetry of the molecular orbitals in both HOMO-LUMO situations is appropriate for a bonding setup and the formation of two sigma bonds.
In general, the most common situation is that of the diene acting as the electron donor from its HOMO and the olefin, the so-called dienophile, as the electron receptor to its LUMO.
This situation is considered as normal electronic demand.
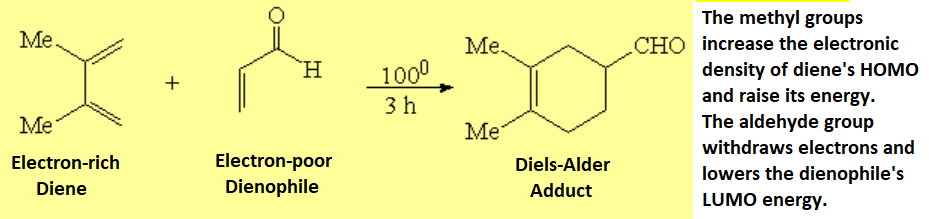
The reaction is thus favored if the diene bears electron-releasing groups and the dienophile electron-withdrawing groups.
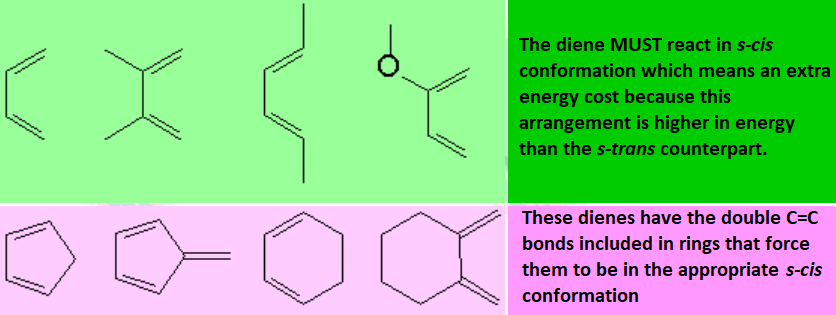
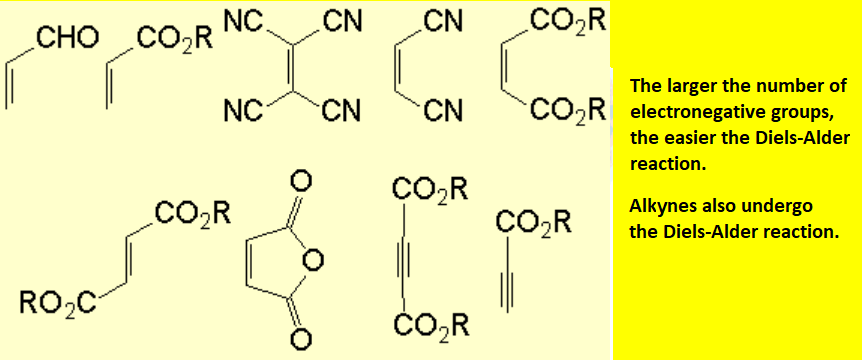
These are examples of very common dienes:
These are instances of usual dienophiles:
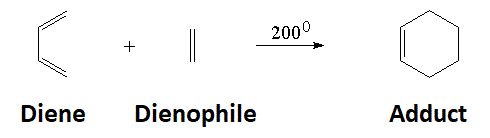
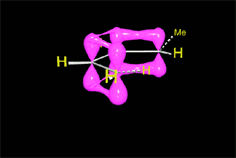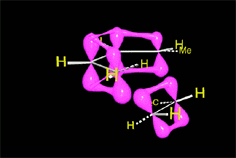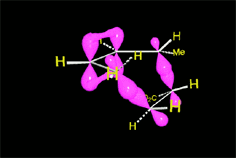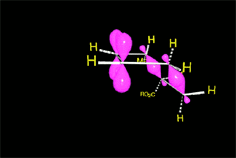
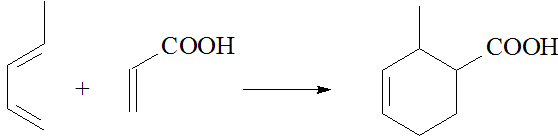


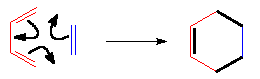 In the single TS the pi electrons circulate leading to the new bonds (in black).
In the single TS the pi electrons circulate leading to the new bonds (in black).