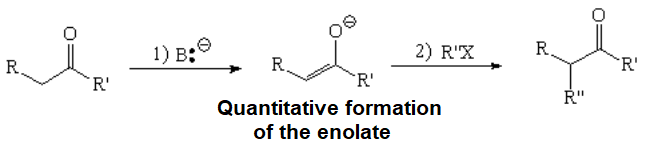
Negatively charged enolates, with an obvious electron excess, are very good nucleophiles that produce nucleophilic substitution over an appropriate compound bearing a good leaving group.
The outcome of this reaction is the alkylation of an aldehyde or ketone at their alpha position, thus increasing the complexity of the newborn molecule relative to the starting one.
The base must be strong enough in order to quantitatively transform the initial aldehyde or ketone into its enolate.
Bases customarily used:
Metal hydrides (NaH)
Alkoxides (t-BuOK)
Metal amides [NaNH2 o LiN(i-Pr)]
The solvent employed has to be inert like dimethoxyethane (DME), dimethyl formamide (DMF), tetrahydrofurane (THF) and hexamethylphosphorotriamide (HMPT).
This reaction shows some issues:
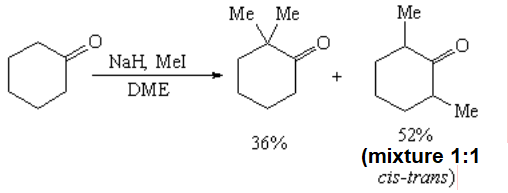 Polyalkylation of symmetric ketones is difficult to avoid, even using one equivalent of alkylating agent.
Polyalkylation of symmetric ketones is difficult to avoid, even using one equivalent of alkylating agent.
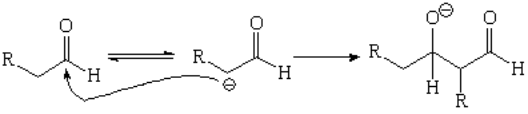 Aldehydes, instead of alkylating, usually react with themselves through a reaction that will be seen in the sequel: aldol condensation.
Aldehydes, instead of alkylating, usually react with themselves through a reaction that will be seen in the sequel: aldol condensation.
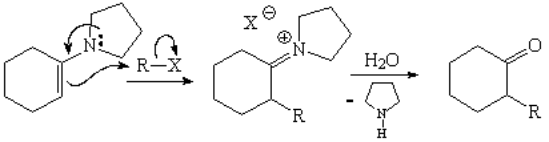
The best way to skip these problems and get regioselective monoalkylations is the use of enamines.
From now on we can regard enamines in a more practical perspective: just like as if they were stable enolates without formal charge, whose formation does not involve the presence of a strong base.
Enamines are less reactive than enolate ions and allow us to perform regioselective monoalkylations skipping the aforementioned problems.
Use ALWAYS enamines whenever you wish to carry out the alkylation of an aldehyde or ketone!!!
 Polyalkylation of symmetric ketones is difficult to avoid, even using one equivalent of alkylating agent.
Polyalkylation of symmetric ketones is difficult to avoid, even using one equivalent of alkylating agent.
 Aldehydes, instead of alkylating, usually react with themselves through a reaction that will be seen in the sequel: aldol condensation.
Aldehydes, instead of alkylating, usually react with themselves through a reaction that will be seen in the sequel: aldol condensation.