PHYSICAL AND BOND PROPERTIES OF UNSATURATED CARBONYL COMPOUNDS
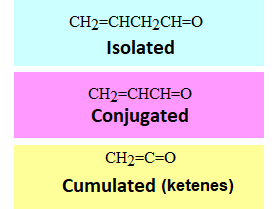
Double C=C and C=O bonds separated by one or more sp3 carbons do not show any different properties to those of the isolated ones.
However, the cumulated and conjugated ones do, similarly to what happens in dienes.
Cumulated Double Bonds (Ketenes)
In order to explain the structure of a ketene (cumulated double bonds), the central carbon must be considered as sp hybridized, NOT sp2.
 The higher "s" character of the hybrid "sp" orbitals of the central carbon make the double C=X bonds shorter than those of plain alkenes and aldehydes or ketones.
The higher "s" character of the hybrid "sp" orbitals of the central carbon make the double C=X bonds shorter than those of plain alkenes and aldehydes or ketones.
Alpha-Beta Unsaturated Carbonyls
The conjugation between C=C and C=O bonds is similar to that of two C=C bonds save from the electron-withdrawing effect of the C=O group.
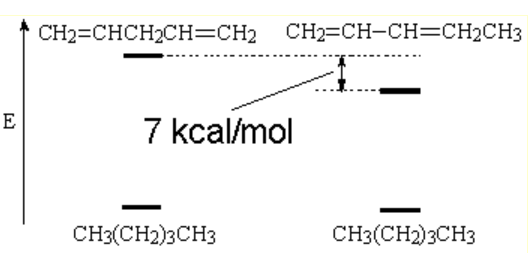
The hydrogenation of 1,3-pentadiene (conjugated diene) happens to be less exothermic than that of its 1,4-isomer (isolated diene) meaning that the conjugated diene is more stable than anticipated.
The difference is ca. 7 cal/mol.
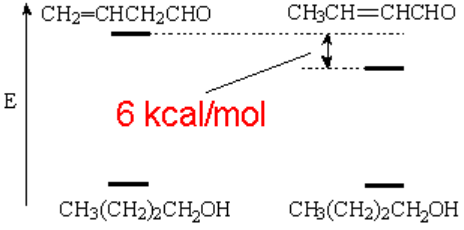
When 2-butenal (conjugated double bonds) is hydrogenated the reaction cedes less energy than its isomer 3-butenal (isolated double bonds).
This fact reveals that the conjugated 2-butenal is ca. 6 kcal/mol more stable.
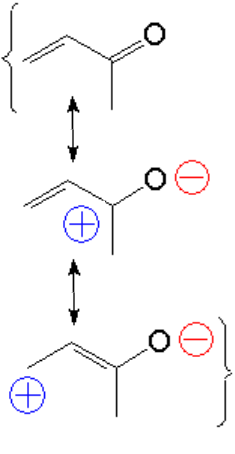
The stabilization gained when the C=C and C=O bonds conjugate is explained in a similar way as when two double C=C bonds do.
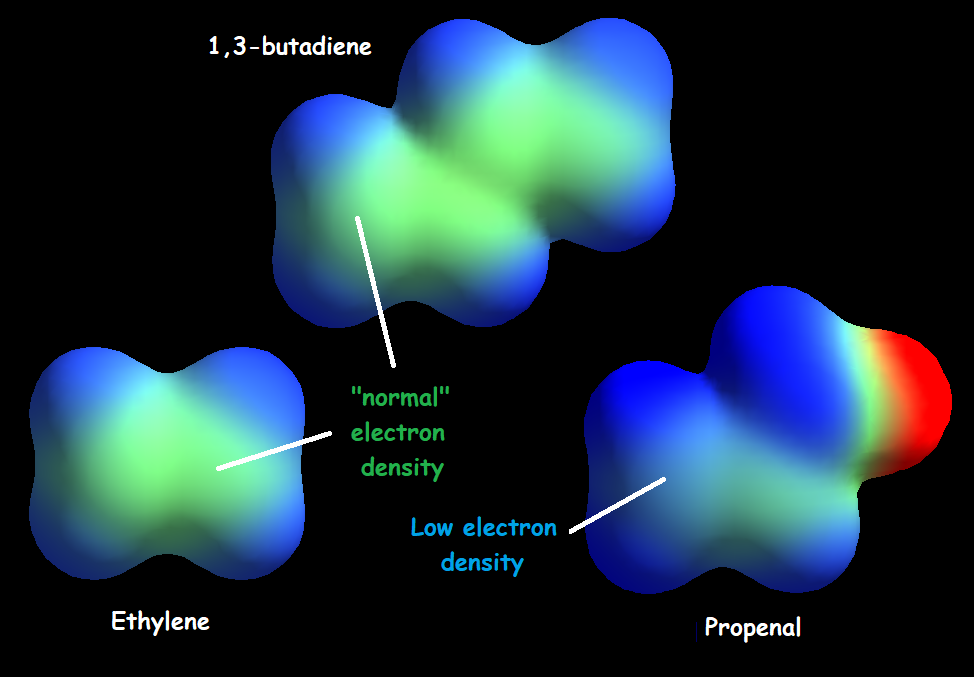
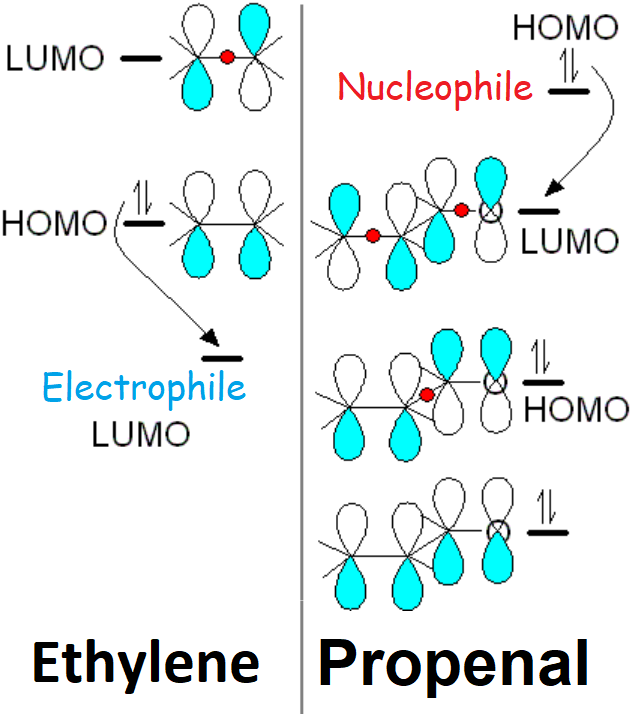
However, the carbonyl C=O group heavily diminishes the electron density of the double C=C bond that can thus be attacked by nucleophiles, just the opposite to what happens with a "normal" double C=C bond.
In alpha,beta-unsaturated carbonyl compounds, both the conjugation and the electronegativity of the oxygen atom induce a stabilization of the "pi" molecular orbitals relative to those of a "normal" alkene.
Consequently, alpha,beta-unsaturated carbonyl compounds react through their electron-empty LUMO with an electron-full nucleophile's HOMO that gives up electrons. On the contrary, a "normal" alkene holds its LUMO too high in energy and then reacts through its HOMO, ceding electrons towards an electrophile's LUMO that receives the electrons.
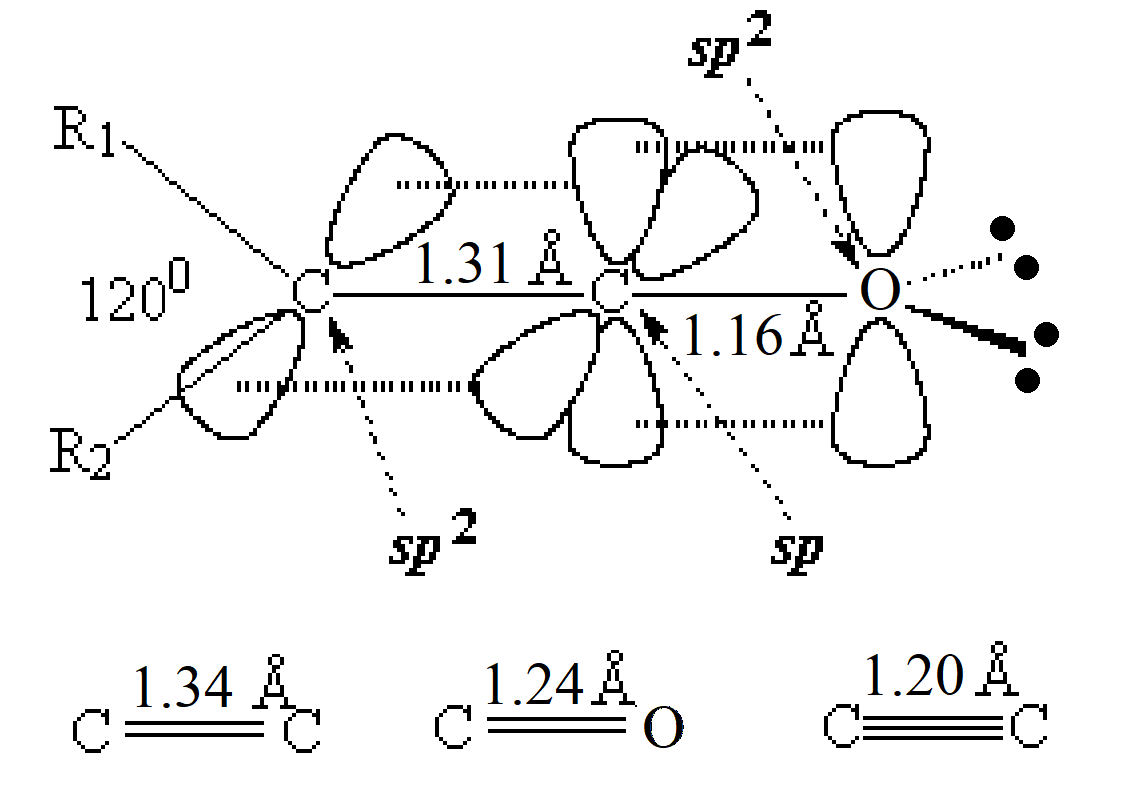
 The higher "s" character of the hybrid "sp" orbitals of the central carbon make the double C=X bonds shorter than those of plain alkenes and aldehydes or ketones.
The higher "s" character of the hybrid "sp" orbitals of the central carbon make the double C=X bonds shorter than those of plain alkenes and aldehydes or ketones.